Subsection 10.3.5 Triple Point of Water
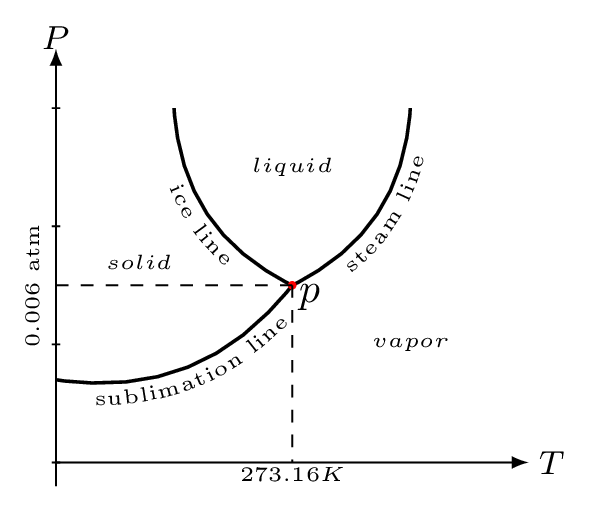
It is a point in the phase diagram at which the solid, liquid, and vapor can co-exist. Phase diagram is characteristic curves of the material between pressure, volume, and temperature. Figure 10.8 is a PT curve shown as a phase diagram of water at constant volume. The of temperature at which the solid and liquid states are in equilibrium is called melting point and the line at which they co-exist is called the ice line. The temperatures at which the solid and gaseous states are in equilibrium is called sublimation point and the line at which they co-exist is called the sublimation line or hoar-frost line. The values of these temperatures change with the pressure. The steam line shows the variation of boiling point of water with the pressure. Ice line shows the variation of melting point of water with the pressure. The negative slope of this line shows the melting point of ice decreases with the increase of pressure. Sublimation line or Hoar-frost line, shows the equilibrium between the ice and steam. These three lines meet at a single point ’p’ called the triple point of water. The triple point of water is 273.16 K at pressure 0.006 atm pressure.
