Subsection 10.3.4 Calorimetry
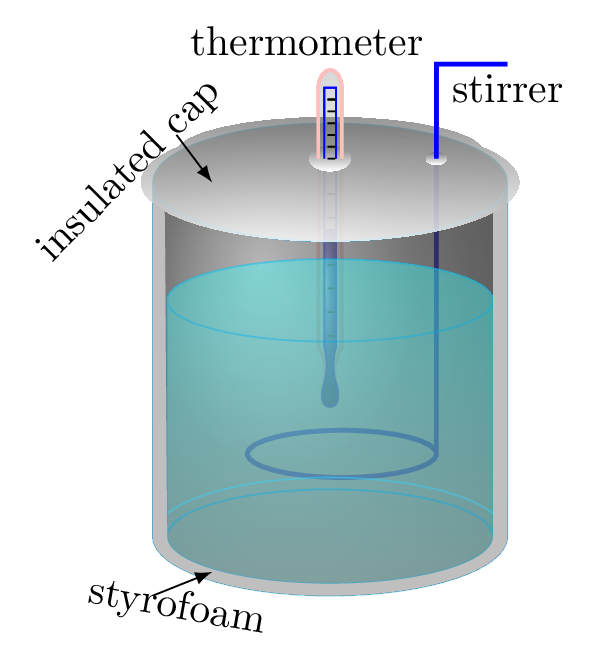
It is a process to measure amounts of heat (calories) transferred to or from a substance. A device used to measure the amount of heat involved in the process is called a calorimeter. A basic calorimeter consists of a metal container of water, in which a thermometer is used to measure the change in water temperature and a stirrer to stir water frequently to maintain uniform temperature. The principle of calorimetry states that in an isolated system heat lost by one component of calorimeter is equal to heat gained by the another components of the calorimeter. A calorie is the amount of energy required to raise one gram of water by one degree Celsius. From the principle of calorimetry,
\begin{equation*}
\sum Q = 0
\end{equation*}
i.e., heat loss = heat gain.
