Subsection 10.3.3 Phase Changes of Matter
We know that ice is solid water, even steam is water as well. Any material can exist in three different states solid, liquid, and gas are also known as the phases of matter. When ice is heated it melts into liquid water and then evaporates into water vapor. These changes from one phase to another are referred to as phase changes.
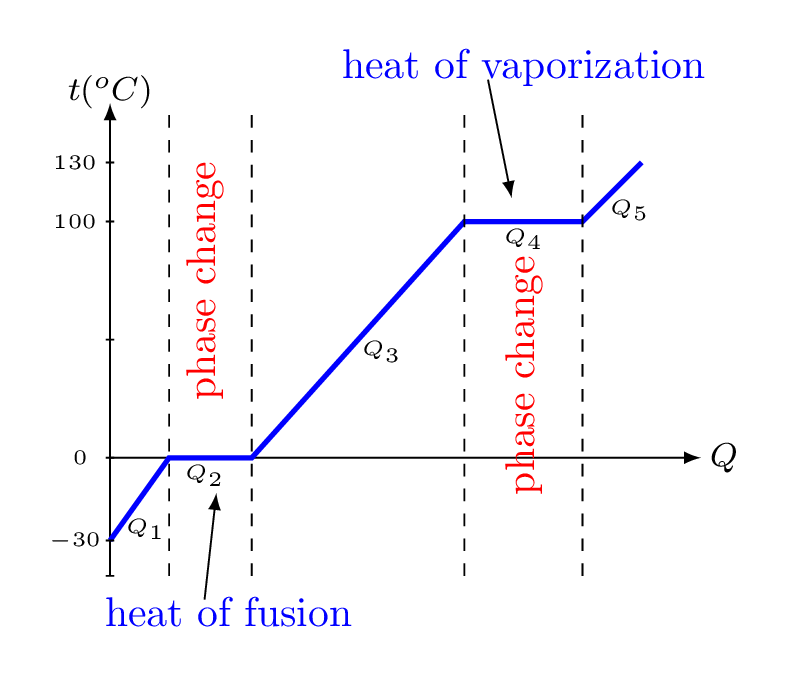
We may also have noticed that the temperature of water doesn’t change during boiling. The temperature of water increases up to boiling and then remains constant as it boils. The following diagram illustrates the relationship between temperature and amount of heat needed to change the phases of water. We can see that the temperature increases as heat is added to water within a phase. Adding heat into the water increases the kinetic energy of water molecules within the ice. During the phase change, the added heat doesn’t make the molecules move faster, but make them further apart. The heat energy added during the phase change is consumed to overcome intermolecular force of attraction and stored within the material in the form of potential energy of the molecules. Where amount of heat added to the system can be obtained by \(Q_{1}= (mc\Delta T)_{ice},\) \(Q_{2}= (mL_{f})_{ice\to water}, \) \(Q_{3}= (mc\Delta T)_{water},\) \(Q_{4}= (mL_{v})_{water\to vapor},\) and \(Q_{5}= (mc\Delta T)_{vapor}.\)
While cooling process heat is taken out from the system, then latent heat of vaporization becomes latent heat of condensation and latent heat of melting becomes latent heat of fusion. For water, latent heat of melting and latent heat of fusion are same. Similarly, latent heat of vaporization and latent heat of condensation are same but they are not same for many other materials. Phase of matter changes from solid to liquid is called melting or fusion, from solid to gas is called sublimation, from liquid to solid is called freezing, from liquid to gas is called evaporation or boiling, from gas to solid is called deposition, from gas to liquid is called condensation, from plasma state to gaseous state is called recombination, and from gaseous state to plasma state is called ionization.
