Section 10.6 Thermodynamics
The word thermodynamics was coined by Lord Kelvin in 1849. It is a combination of two words, thermo that refers heat and dynamics that refers force or motion of the molecules or atoms. It is the branch of physics which deals with heat energy, its transfer, and energy conversion. It begins its journey with the development of fire. People used to believe that every substance has some amount of invisible fluid called caloric fluid. When it moves from one object to another the object becomes hot or cold. The object which loses caloric fluid becomes cold and which gains caloric fluid becomes hot. We know now that there is nothing like caloric fluid but it is a heat energy which exists in a material due to molecular vibration. Heat is a form of energy that can flow due to temperature difference. We can obtain an infinite amount of heat energy out of the material. For example, we can produced an infinite amount of heat from water by performing a continuous mechanical work (as in Joul’s experiment Figure 10.6.3.(a)) without changing the condition of water. Work is also an energy that is transferred from one system to another without considering the temperature difference between them.
There are several processes in nature which occur always in a particular fashion. For examples, water falls from a high elevation to low elevation, heat flows from high temperature to low temperature, current flows from high electric potential to low electric potential, in diffusion molecules flows from high concentration to low concentration, we grow older not younger, our hair turns gray not black as we go older, and so on. Even in the conversion of mechanical energy into heat energy, for example if you rub your right hand finger on the palm of left hand (mechanical work) you produce a heat but reverse does not seem to happen. Yes, some events can happen in both directions, for example you can heat a gas and you can cool a gas, you expand a gas and compress a gas, etc but even in that processes we can see there are some changes happen in the surroundings. We will discuss those events later in second law of thermodynamics which talks about the directional property of heat energy and its conversion. The conversion of mechanical energy into electrical energy and vice a versa is 100% possible in ideal case but if we want to convert heat energy into mechanical work (energy) then 100% conversion is not possible even in the ideal case. However, we can convert all of our mechanical energy into heat energy. Hence we can tell that the nature always follows a certain pattern to have some process to occur. Nature obeyes simple and universal laws that can be expressed in the mathematical language. Thermodynamics is thus a fundamental subject of nature that describes the basic laws governing the physical process and its occurrence associated with the transfer of energy and its conversion. It is based on our common experience, observation, and experimentation. It has two parts in observational points of view, that is microscopic view (statistical thermodynamics) and macroscopic view (classical thermodynamics). The characteristic of the over all behavior the system can be sensed in classical thermodynamics. The behavior of system on the basis of its molecular property can be studied in statistical thermodynamics. That is, the classical thermodynamics is an average outcome of statistical thermodynamics. Thermodynamics provides a very important aspects of our life that tells us which process in nature can only occur and which process can not occur.
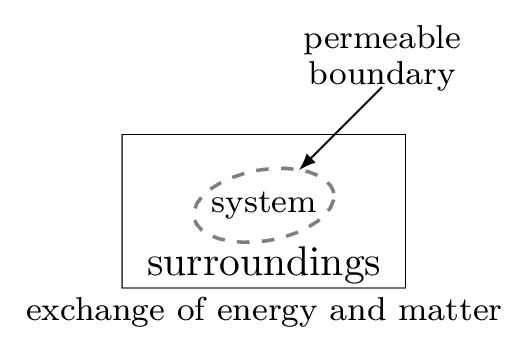
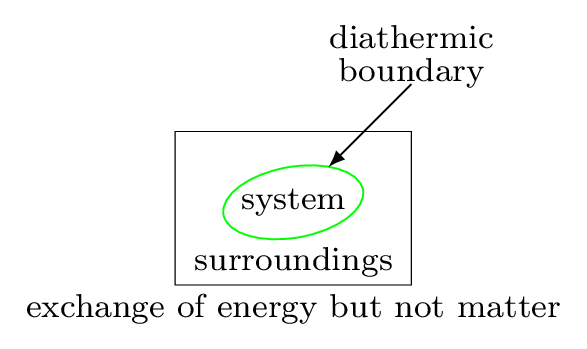
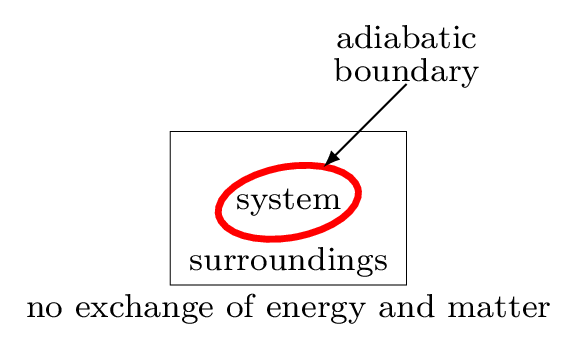
To understand the thermodynamical process we need to know about the system and surroundings. The system is the part of the universe being studied, while the surroundings are the rest of the universe that interacts with the system. The system and surroundings are separated by a hypothetical line called a boundary. It may be real or imaginary, for example water in a beaker is separated from rest of the room, then the beaker behaves as a boundary, water as a system, and the rest of the room as surroundings. In other word, a system is a definite amount of matter bounded by a closed surface. Anything outside of this closed surface is called a surroundings. The closed surface is a boundary which separates the system and the surroundings. The boundary may be real or imaginary, it may be fixed, flexible, or distorted. System and surroundings must interact with each other by exchanging energy of any forms. System which can exchange energy and matter both with the surroundings is called open system. For example, boiling water in an open pot. If the system can exchange only energy but not the matter with its surroundings then system is called a closed system. For example, a cup of coffee with a lid on it. Some system neither exchange energy nor the matter with its surroundings then it is called an isolated system. For example, a thermos flask.
