Subsection 10.6.2 Laws of Thermodynamics
The laws of thermodynamics define fundamental physical quantities such as temperature, energy, and entropy that characterize thermodynamic systems. There are four laws of thermodynamics.
Subsubsection 10.6.2.1 Zeroth Law of Thermodynamics
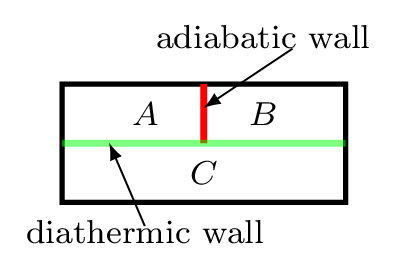
It defines the temperature and states the thermal equilibrium condition. Thermal equilibrium is a state achieved by two or more systems after being in contact with one another through a diathermic wall. If an object with a higher temperature comes in contact with another object at a lower temperature, it will transfer some of its heat energy to the object at lower temperature till they come to the same temperature. If two systems are each in thermal equilibrium with a third system, then they must be in thermal equilibrium with each other. If temperature of object A = temperature of object C and temperature of object B = temperature of object C, then temperature of object A = temperature of object B [Figure 10.6.2].
Subsubsection 10.6.2.2 First Law of Thermodynamics
The first law is a consequence of conservation of energy and requires that a system may exchange energy with its surroundings strictly by heat flow or work. Joule has established the equivalence between heat and mechanical work as
\begin{equation*}
W=JQ,
\end{equation*}
where W is amount of work done (in joule) on the system, Q is the amount of heat (in calorie) produced in the system, and J is a mechanical equivalent of heat also known as Joule’s constant. The value of \(J\) is \(4.1860 \,J/cal\text{.}\) The first law states that when a given amount of work, W is done on a system, an equivalent amount of heat, Q is produced or when a certain amount of heat, Q disappears from the system an equivalent amount of work W is obtained. The equivalence is given by the constant \(J\text{.}\) Heat is thus a form of energy.
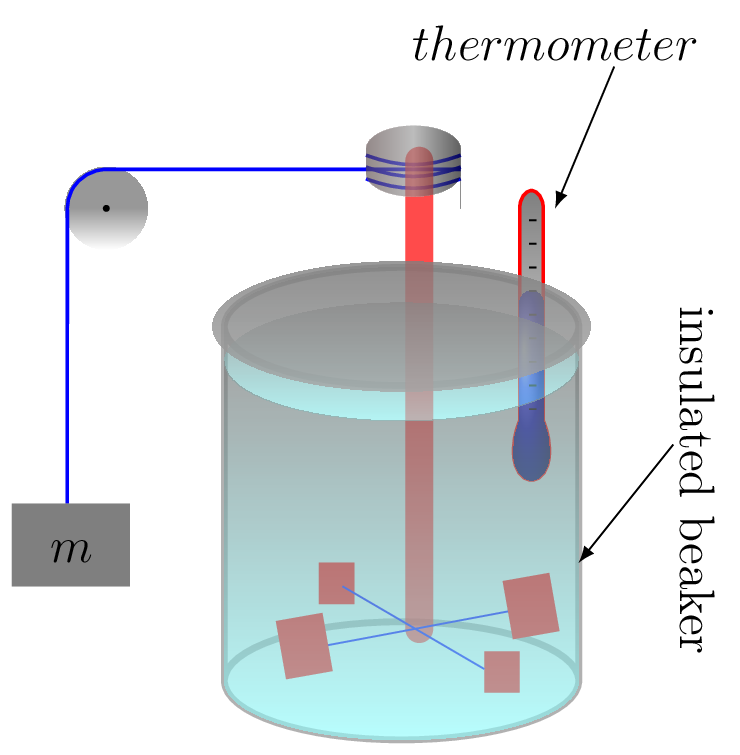
The Joule apparatus [Figure 10.6.3.(a)] consisted of a weight suspended by string over a pulley, which in turn was wound around a winding drum. As long as the drum remained stationary, the weight remained at rest. When the drum was released, the weight was set free to fall, and its potential energy began converting to kinetic energy. In the process, the string attached to the weight unwound from the drum, which caused the drum to turn and along with it the paddle wheel attached to it. The rotating paddles agitated the water, causing its temperature to rise. Joule concluded from this experiment that the mechanical energy of the spinning paddle wheel had been converted into heat energy, which raise the temperature of the water. Joule’s experiment thus proved the link between potential, kinetic, mechanical, and heat energies. Joule’s experiment thus helped establish the principle of conservation of energy.
Let us consider a system which absorbed \(Q\) amount of heat and does \(W\) work done while going from initial state \(i\) to the final state \(f\text{.}\) The final state reached by the system may be achieved by the different paths but the quantity \(Q-W\) is the same. The quantity \(Q-W\) is known as the change in internal energy \(\Delta U \) of the system.
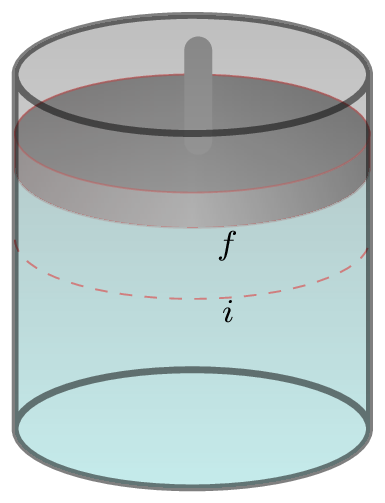
Consider a gas cylinder [Figure 10.6.3.(b)] with frictionless piston placed on a hot bath, then the change in internal energy of the cylinder is given by
\begin{equation}
\,dU = \,dQ-\,dW \tag{10.6.1}
\end{equation}
where \(\,dQ \) is the amount of heat energy absorbed by the gas, and \(\,dW\) is the amount of work done by the gas in moving its piston from position \(i\) to \(f\text{.}\) This is the mathematical form of I law of thermodynamics. The I law establishes an exact relation between heat and work. This tells us that it is impossible to get the work from any machine without giving it an equivalent amount of energy in any form.
Quasi static state: A process during which the system only deviates from equilibrium by an infinitessimal amount. This may happen if the gas is allowed to expand or compress very slowly. As the piston compresses the pressure inside the gas remains very nearly uniform at all times during the process. So, the system never deviates significantly from mechanical equilibrium. Quasi static process is also a reversible process. A system is in thermodynamical equilibrium if it has achieved: mechanical equilibrium, chemical equilibrium, and thermal equilibrium. Thermodynamic variables like temperature, pressure etc. can only be used to describe the system that has achieved thermodynamical equilibrium.
