Subsection 10.1.2 Absolute Scale
A physical thermometer uses the physical properties of a material or substance to determine temperature. It relies on the fact that certain physical properties change in a predictable way with temperature. Examples of physical thermometers include mercury-in-glass thermometers, alcohol thermometers, and bimetallic strip thermometers. These thermometers use the expansion and contraction of materials (e.g., mercury or alcohol) to indicate temperature changes. The scale of normal thermometers depends upon the physical propertise (thermal expansion, electrical resistance, etc) of the substance which is used in the thermometer. The physical property (e.g. thermal expansion) of materials varies differently at the different range of temperatures hence all these thermometers have fixed uses. For example, a mercury thermometer can measure temperature ranges from approximately \(-39^oC \) to \(356^oC\) (\(-38^oF\) to \(672^oF\)). For alcohol thermometer, the typical temperature range is approximately \(-115^oC\) to \(78^oC\) (\(-175^oF\) to \(172^oF\)). That means, if we go to Antarctica we must bring with us an alcohol thermometer and when going to Lut Desert a hotest place on earth, we need a mercury thermometer. Mercury thermometer may freeze in Antarctica and alcohol thermometer may boil in Lut Desert. To remedy such problems of limitation of physical thermometers, Scottish physicist William Thomson, \(1^{st}\) Baron Kelvin proposed to constructing a temperature scale which should be independent on the material properties on the basis of thermodynamics principles that the lowest possible temperature which could be achieved was \(-273^{o}C,\) in 1848. This minimum temperature is the zero point on Kelvin scale and is known as absolute zero. Kelvin scale is a hypothetical (theoretical) thermometer scale also known as absolute scale.
1
From third law of thermodynamics, it is impossible to reach absolute zero temperature by any physical means.
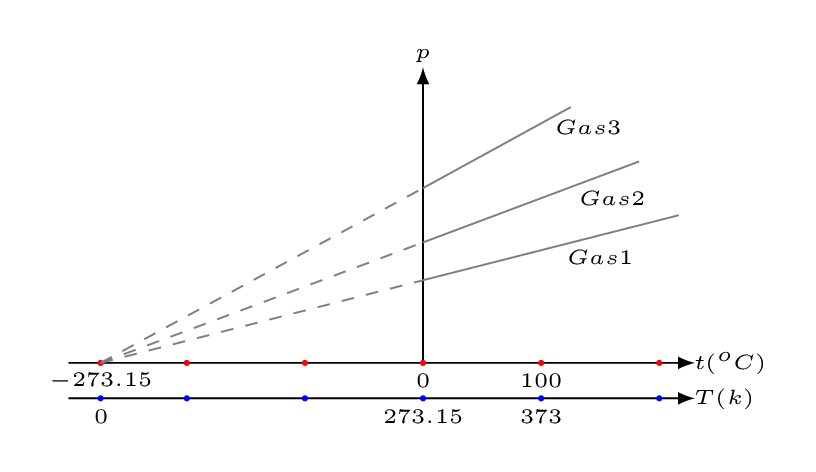
Temperature is a measure of average kinetic energy per molecule of any substance. In classical mechanical point of view, molecular motion ceases completely at absolute zero and hence possesses zero kinetic energy. (However, in quantum mechanical point if view, at absolute zero molecular motion takes minimum speed of its vibration.) It has been seen from the gas laws that the product of pressure and volume is directly proportional to temperature [Figure 10.1.2]. In fact, all gases have energy (\(pV\)) heading to zero at the same temperature called absolute zero. At absolute zero the pressure of an ideal gas would drop to zero.
