Subsection 10.6.4 Second Law of Thermodunamics
The first law of thermodynamics gives an equivalence between the quantity of heat and the mechanical work or vice-versa. This law does not tell the limitation and condition of conversion. That is how much heat is converted into work and whether the transformaiton itself can take place or not. The first law does not tell anything about the direction of transformation of energy. It does not indicate whether heat can flow from a cold end to a hot end or not. The first law places no restriction on the direction of a process, and satisfying the first law does not guarantee that the process will occur. In practice, it is not possible to convert the heat energy into an equivalent amount of work. Thus, we need another general principle to identify whether a process can occur or not. The law specifying the condition of transformation of heat into work is called the second law of thermodynamics. The second law of thermodynamics tells the direction of the flow of heat. It also tells that heat energy cannot be completely converted into equivalent work.
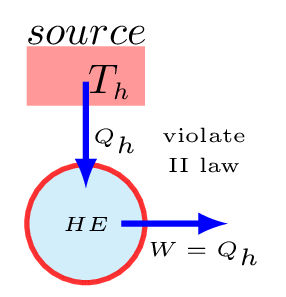
Kelvin-Plank statement of the second law: It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work [Figure 10.6.4.(a)]. For heat engine to operate, the working fluid has to exchange heat with heat sink as well with the heat source. A heat engine that violates the Kelvin-Planck statement of the second law cannot be built.
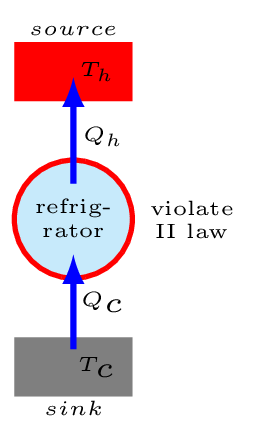
Clausius statement of the second law: It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a cold body to a hot body. In other words, a refrigerator will not operate unless an external work is done on its compressor [Figure 10.6.4.(b)].
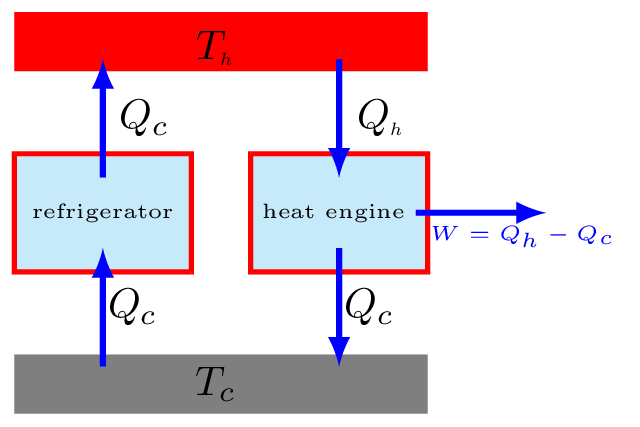
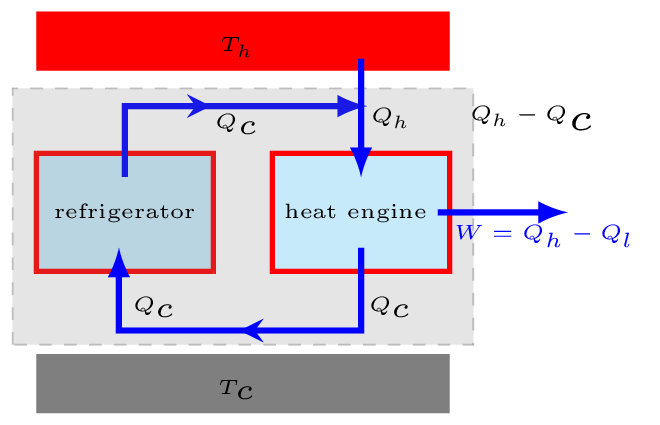
The above two statements are equivalent to each other. Consider a refrigerator that transfers heat \(Q_{c}\) from a cold to a hot body without having any work done on it [Figure 10.6.4.(c)], thus violating the Clausius statement. Now, suppose an engine working between the same hot and cold bodies takes in heat \(Q_{h}\) from a hot body and gives out heat \(Q_{c}\) to the cold body [Figure 10.6.4.(c)]. The engine does not violate any law by itself, but if the refrigerator and engine combined together [Figure 10.6.4.(d)], they form a device that takes in heat \(Q_{h}-Q_{c}\) from the hot body and converts all into work without delivering any to the cold body. This is a violation of Kelvin’s statement. Similarly, a violation of the Kelvin’s statement leads to violation of the Clausius statement.
