Subsection 8.1.1 The Classification of Matter
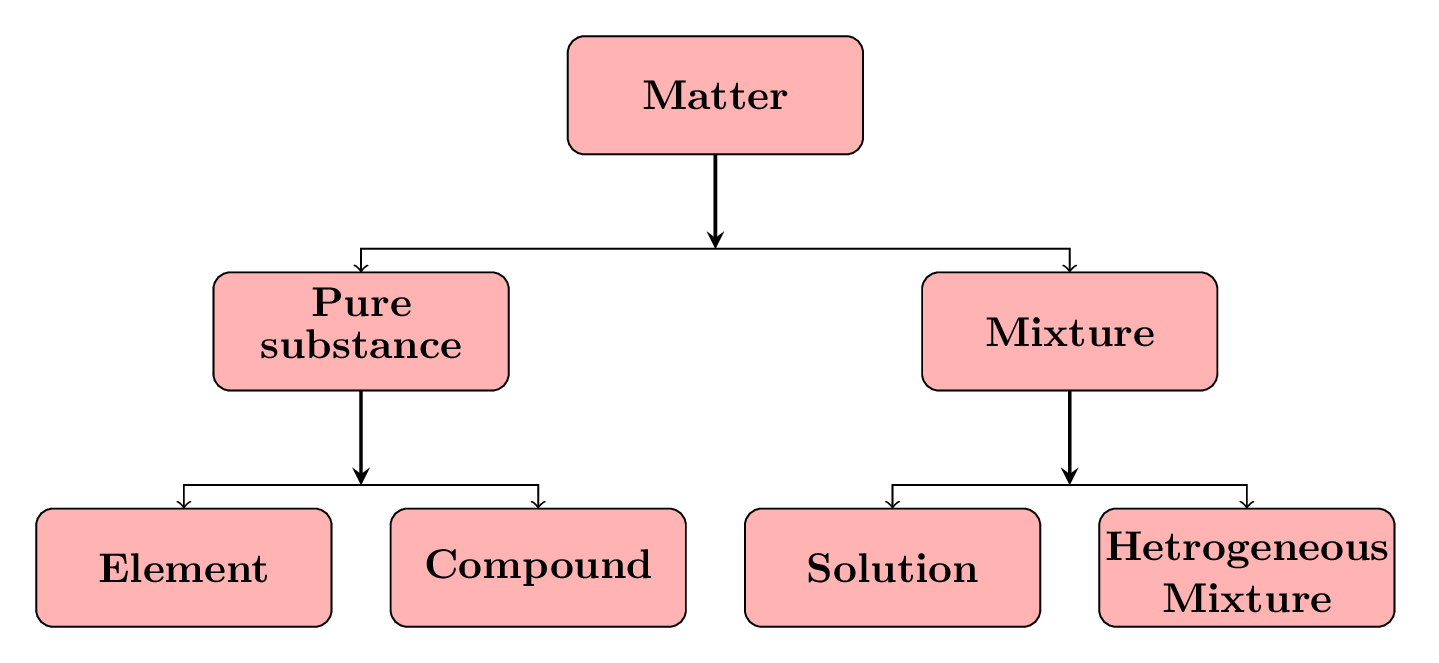
Instead of three phases (or states) of matter, there are three different classes of matter based on its composition and properties. They are elements, compounds, and mixture Figure 8.1.1.
- Elements: An element is a pure substance that cannot be broken down into simpler substances by any chemical means. Elements are the building blocks of all matter and are composed of only one type of atom. Examples of elements include hydrogen, oxygen, and gold.
- Compounds: A compound is a pure substance that is made up of two or more elements chemically combined in a fixed ratio. Compounds have different properties from their constituent elements and cannot be separated into their individual elements by physical means. Examples of compounds include water (\(H_2O\)), salt (\(NaCl\)), and glucose (\(C_6H_{12}O_6\)).
- Mixtures: A mixture is a combination of two or more substances in which each substance retains its own chemical properties. Mixtures can be separated into their individual components by physical means. There are two main types of mixtures: homogeneous mixtures, where the substances are evenly mixed, and heterogeneous mixtures, where the substances are not evenly mixed. Examples of mixtures include air, fruit juice, and concrete.
In homogeneous mixture substances are evenly mixed and have the same composition throughout. Homogeneous mixture is also called a solution Here are some examples of homogeneous mixtures: Salt water: When salt is dissolved in water, the result is a homogeneous mixture, in which the salt and water are evenly distributed throughout the solution. Air: Air is a mixture of gases, including nitrogen, oxygen, and trace amounts of other gases, that are evenly mixed and have the same composition throughout the entire volume of air. Honey: Processed honey is a homogeneous mixture but raw honey is not. Vinegar: Vinegar is a homogeneous mixture of water and acetic acid, with the water and acetic acid evenly mixed throughout the entire substance. Saltwater, Sugar solution, Seawater, Soda Pop, etc are some examples of homogeneous mixture.
In heterogeneous mixture substances are not evenly mixed and have different compositions in different parts of the mixture. Heterogeneous mixtures are easy to identify, as the individual substances are easily visible and can be separated from one another by physical means. Here are some examples of heterogeneous mixtures: Fruit salad: A mixture of fruit, such as apples, bananas, and grapes, cut into pieces and combined in a bowl is a heterogeneous mixture. Each piece of fruit has its own unique composition, and the composition of the mixture changes from one part of the mixture to another. Granola: A mixture of oats, nuts, dried fruit, and sweeteners is a heterogeneous mixture, as the ingredients are not evenly mixed and the composition of the mixture changes from one part of the mixture to another. Sand and water: A mixture of sand and water is a heterogeneous mixture, as the sand particles settle to the bottom of the container and the water is clear. The composition of the mixture changes from the top of the mixture to the bottom. Soil: Soil is a mixture of minerals, organic matter, air, and water, and is a heterogeneous mixture as the composition of the soil changes from one part of the soil to another. Concrete: Concrete is a mixture of cement, sand, gravel, and water, and is a heterogeneous mixture, as the composition of the concrete changes from one part of the mixture to another.
Milk is neither a homogeneous nor a hetrogeneous mixture rather it comes under coloidal solution type. Milk is a mixture of water, fat, proteins, and sugars that are not evenly distributed throughout the mixture. Blood is also a coloidal solution. Fog, gelatin, smoke, etc. are some examples of coloidal type. Colloids have larger particles that are dispersed in a medium non-uniformly at the molecular level.
