Subsection 11.1.1 Composition of Earth’s Crust
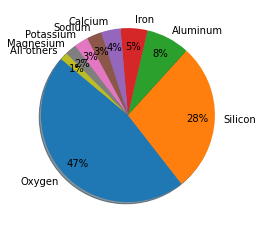
The Earth’s crust is composed of different elements and minerals. The most abundant elements in the Earth’s crust are oxygen (~47%), silicon (~28%), aluminum, iron, calcium, sodium, potassium, and magnesium. These elements combine to form a variety of minerals, such as quartz, feldspar, mica, and olivine. The exact composition of the Earth’s crust varies depending on factors such as location and geological history, but in general, the crust is composed of three main types of rocks: igneous, sedimentary, and metamorphic.
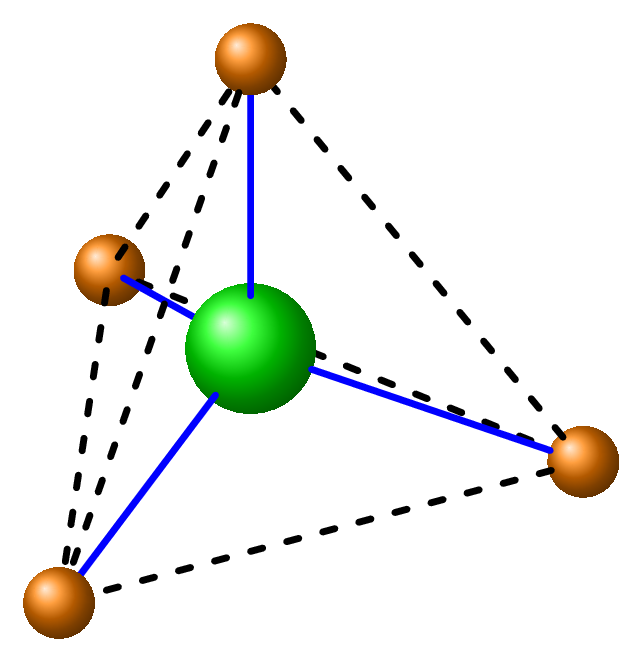
Silica, or silicon dioxide \((SiO_2)\text{,}\) and Silicates (combination of silicon, oxygen, and other metals such as aluminum, iron, and magnesium, \(CaSiO_3\)) are the most abundant compounds of the Earth’s crust. For example, quartz, which is composed of silicon dioxide, is one of the most common minerals in the Earth’s crust. When rocks containing silica are exposed to the elements, such as wind and water, the silica can be broken down and transported to other locations, where it can form new rocks. Slicates are an important components of many types of rocks, including igneous, sedimentary, and metamorphic rocks. In igneous rocks, silicates are the dominant mineral group, and they are the primary component of rocks such as granite and basalt. In sedimentary rocks, silicates are also abundant and can be found in rocks such as sandstone and shale. In metamorphic rocks, silicates are often transformed into new minerals due to the effects of heat and pressure. Silicates are important in the Earth’s crust because they are involved in many geological processes. For example, the weathering of silicate minerals can release nutrients such as potassium and phosphorus that are essential for plant growth. Whetering hepls silicates to recycle carbon dioxide. The tetrahedron geometry of silicate \((SiO_4^{4-})\) ion is given in the Figure 11.1.2.(b)
