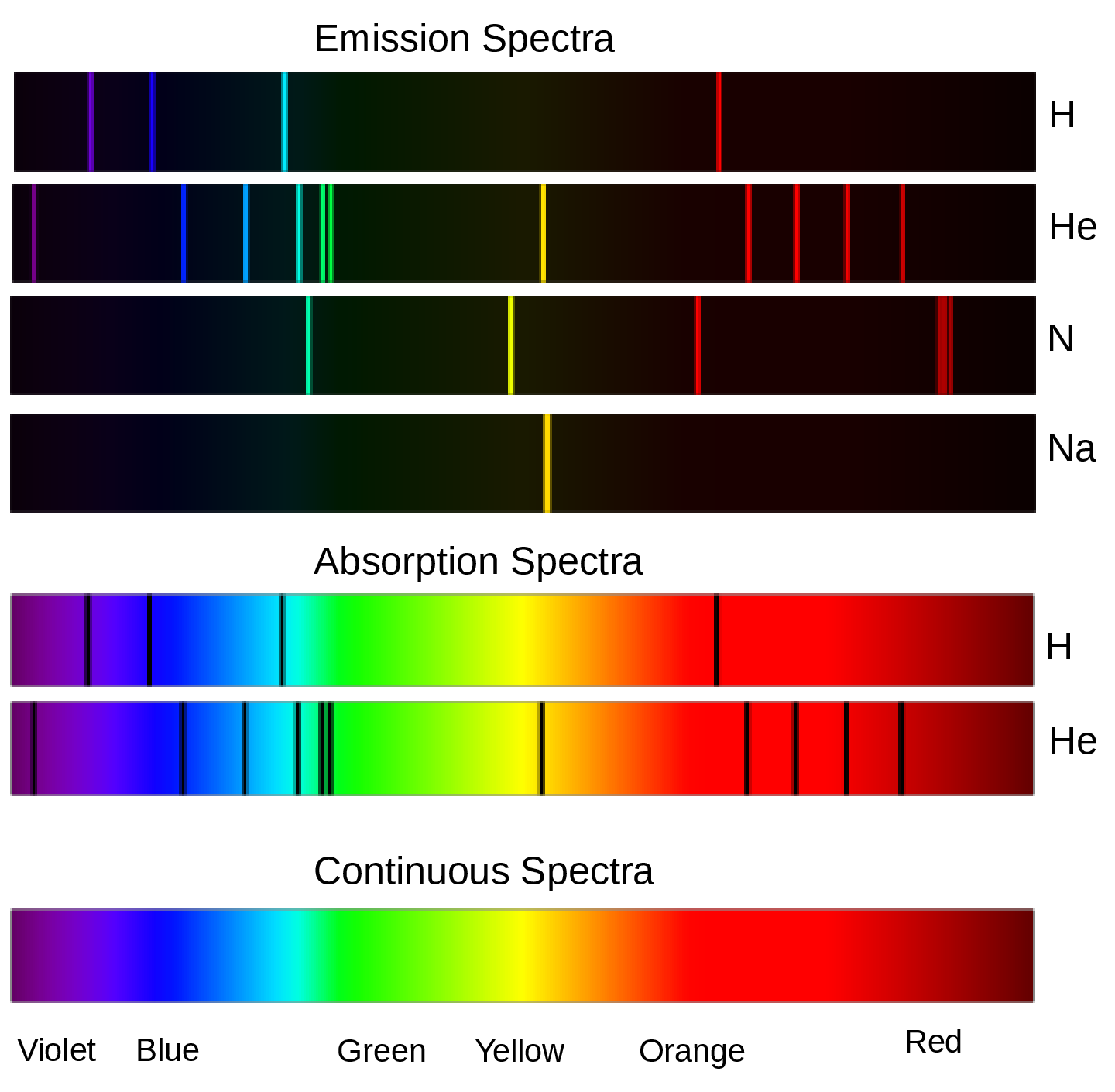
Subsection 7.1.5 Origin of Spectra
The term "spectrum" originated from the Latin word "spectrum" which means "appearance". The idea of a spectrum in physics and optics refers to the distribution of light into its component colors (red, orange, yellow, green, blue, indigo, and violet) when passed through a prism or diffraction grating. Spectral lines are caused by the interaction of electromagnetic radiation with atoms or molecules. When an atom or molecule is excited by absorbing a photon of light, its electrons can transition to higher energy levels. When the electrons eventually fall back down to their ground state, they emit photons of light at specific, characteristic wavelengths, which correspond to the difference in energy between the two states. This results in the emission of light at specific wavelengths, known as spectral lines. The position and intensity of these lines are unique to the elements or compounds present, and can be used to identify and analyze the composition of stars, planets, and other celestial objects. The Figure 7.1.6 is the visible emission spectrum of H, He, and N line spectra, and their absorption spectra.