Section 8.2 The Periodic Table
The periodic table of elements is a tabular arrangement of the chemical elements based on their atomic structure, chemical properties, and electron configurations. The modern periodic table consists of 118 elements and is arranged in rows and columns based on their atomic number and electron configurations. The periodic law states that the physical and chemical properties of elements are periodic functions of their atomic numbers. This means that when elements are arranged in order of increasing atomic number, their properties exhibit a recurring or periodic pattern.
The discovery of the periodic table is generally attributed to the Russian chemist Dmitri Mendeleev, who published his version of the table in 1869. However, other scientists, including John Newlands and Julius Lothar Meyer, also made significant contributions to the development of the periodic table around the same time. Mendeleev’s table was particularly influential because he left gaps for undiscovered elements and predicted the properties of these elements based on their position in the table. When these elements were discovered, their properties were found to match Mendeleev’s predictions, which helped to establish the credibility of the periodic table Figure 8.2.1.
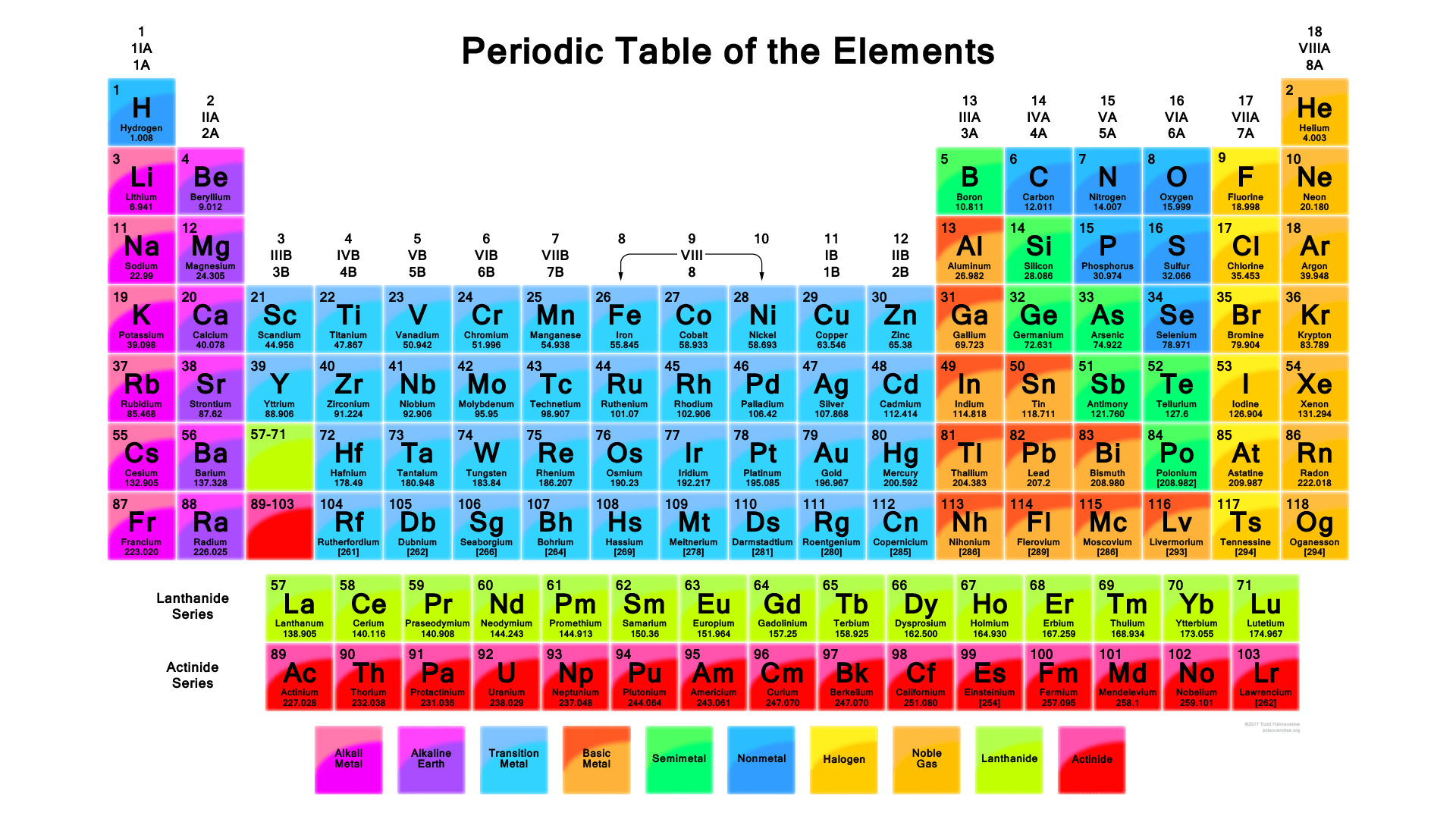
The Periodic Table1
sciencenotes.org/hd-wallpaper-periodic-table/Here is a general guide on how to read the periodic table:
- Element symbols: Each element is represented by a one- or two-letter symbol. For example, "H" represents hydrogen and "He" represents helium.
- Atomic number: Each element’s atomic number is located above or below its symbol. The atomic number represents the number of protons in the element’s nucleus.
- Element name: The name of each element is located below its symbol.
- Groups: Elements with similar chemical properties are organized into vertical columns called groups or families. There are 18 groups in the modern periodic table, numbered 1 to 18 from left to right. Elements in the same group have similar chemical and physical properties.
- Periods: The elements in the periodic table are arranged in horizontal rows called periods. The period number represents the highest energy level or shell occupied by electrons in that element.
- Atomic mass: The average atomic mass of each element is located below its symbol. This value represents the average mass of all the naturally occurring isotopes of that element.
- Blocks: The periodic table is also divided into four blocks, based on the electron configuration of the outermost shell. These blocks are s-block, p-block, d-block, and f-block.
The modern periodic table is divided into two main categories: the "A" groups, also known as the main group elements, and the "B" groups, also known as the transition metals. The A groups, or main group elements, are located in columns 1, 2, and 13 to 18 of the periodic table. These elements are also known as the representative elements, and they are characterized by having valence electrons in the s and p orbitals of their outermost energy level. The A groups are further divided into two subcategories: the alkali metals (Group 1) and the alkaline earth metals (Group 2), which are located on the far left side of the periodic table, and the post-transition metals (Groups 13-16), the halogens (Group 17), and the noble gases (Group 18), which are located on the right side of the table. Rare earth elements (Group 3) are metallic chemical elements that are crucial components of many modern technologies, including smartphones, wind turbines, electric vehicles, and medical devices. These elements are similar in their electronic structure and chemical properties, and they all have partially filled 4f electron shells. Because the electronic properties of these elements are highly similar, they are difficult to separate, which is one reason why rare earth element extraction and refining can be challenging and expensive. Despite their name, rare earth elements are not actually rare. They are found in relatively small quantities in the Earth’s crust, and are often difficult and expensive to extract and refine.
Most post-transition metals are actually is non-metals. Non-metals are elements that do not have metallic properties and are typically poor conductors of heat and electricity. The non-metals are located in the upper right corner of the periodic table and are grouped together in several columns, or groups. The main groups of non-metals in the periodic table are:
- Group 14 (Carbon Group): This group includes the non-metal carbon (C), which is the basis for all organic molecules, as well as the metalloids silicon (Si) and germanium (Ge).
- Group 15 (Nitrogen Group): This group includes the non-metal nitrogen (N), which is a key component of the Earth’s atmosphere, as well as the non-metals phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi).
- Group 16 (Chalcogens): This group includes the non-metals oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po), and livermorium (Lv). The chalcogens are known for their ability to form compounds with a wide range of other elements, and they are involved in many important biological and industrial processes. For example, oxygen is essential for respiration and combustion, while sulfur is used in the production of fertilizers and other chemicals. Selenium and tellurium are used in the manufacture of semiconductors, while polonium has important nuclear applications. They are non-metals, and they have relatively high electronegativities and melting points. They also tend to form compounds with other elements that are covalent or ionic in nature.
- Group 17 (Halogens): This group includes the non-metals fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). The halogens are highly reactive and form a variety of compounds.
- Group 18 (Noble Gases): This group includes the non-metals helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The noble gases are generally unreactive and have very low boiling points.
