Subsection 9.3.1 The pH Scale
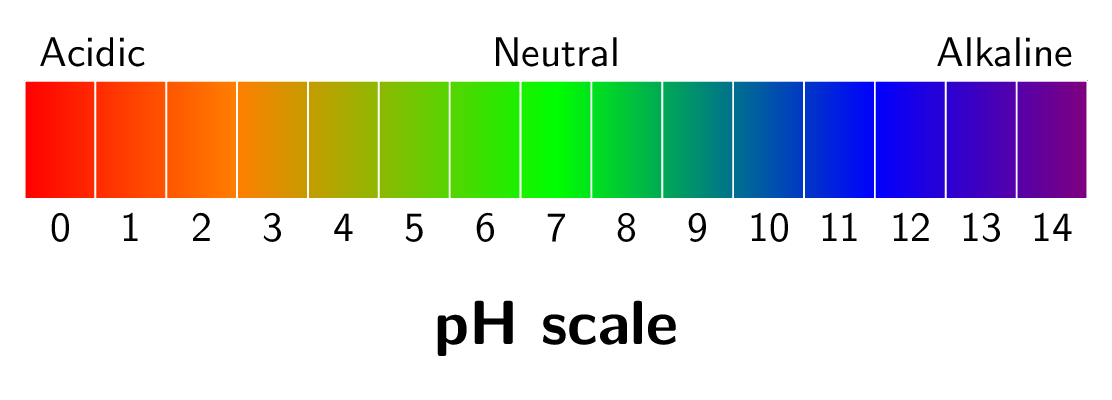
One of the most important properties of acids and bases is their \(pH\) level. The pH scale is a measurement system used to describe the acidity or basicity (alkalinity) of a solution. It is a logarithmic scale that ranges from 0 to 14, with 7 being neutral. A solution with a pH less than 7 is considered acidic, while a solution with a pH greater than 7 is considered basic (alkaline). The strength of an acid or base depends on its ability to release or accept hydrogen ions. Each unit on the pH scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and 100 times more acidic than a solution with a pH of 6. Similarly, a solution with a pH of 10 is ten times more basic than a solution with a pH of 9, and 100 times more basic than a solution with a pH of 8. The pH scale is important in many scientific fields, including chemistry, biology, and environmental science. It is used to measure the acidity or basicity of substances such as water, soil, and blood.
The hydronium ion, also known as the oxonium ion, is a positively charged ion with the chemical formula \(H_3O^+\text{.}\) It is formed when a water molecule, \(H_2O\text{,}\) accepts a hydrogen ion, \(H^+\text{,}\) from an acid. The hydronium ion is an important species in acid-base chemistry because it represents the acidic character of a solution. In aqueous solutions, the concentration of hydronium ions determines the pH of the solution. Solutions with a high concentration of hydronium ions have a low pH and are considered acidic, while solutions with a low concentration of hydronium ions have a high pH and are considered basic (alkaline). Hydronium ion can act as a proton donor (acid) or proton acceptor (base) in chemical reactions, making it an important species in acid-base catalysis. Pure water has some contents of hydronium ion when it dissociates but these ions recombines also very fast, hence, pure water is neutral and has pH scale of 7.
\begin{equation*}
H_2O \longrightarrow H^+ + OH^- \text{dissociation of water}
\end{equation*}
Since hydroxide ion, \(OH^-\) attract \(H^+\) ion strongly there is reverse reaction keep happening in water
\begin{equation*}
H^+ + OH^- \longrightarrow H_2O \text{recombination of water}
\end{equation*}
Thus we can write
\begin{equation*}
H_2O ⇌ H^+ + OH^-
\end{equation*}
