Subsection 7.2.3 Nuclear Reactions
Nuclear reactions are the process in which nucleus of an atom changes. These changes can be either decay processes, in which the nucleus of an atom emits particles or radiation, or fusion processes, in which two or more atomic nuclei come together to form a heavier nucleus. There are two main types of nuclear reactions:
- Nuclear Fission Reactions: These reactions involve the spontaneous decay of an unstable nucleus into a more stable form. Common types of nuclear decay reactions include alpha decay, beta decay, and gamma decay.
- Nuclear Fusion Reactions: These reactions involve the fusion of two atomic nuclei to form a heavier nucleus. This process releases a large amount of energy, and is the reaction that powers the sun. Nuclear fusion reactions are difficult to achieve on Earth, but are being researched as a potential source of clean and abundant energy.
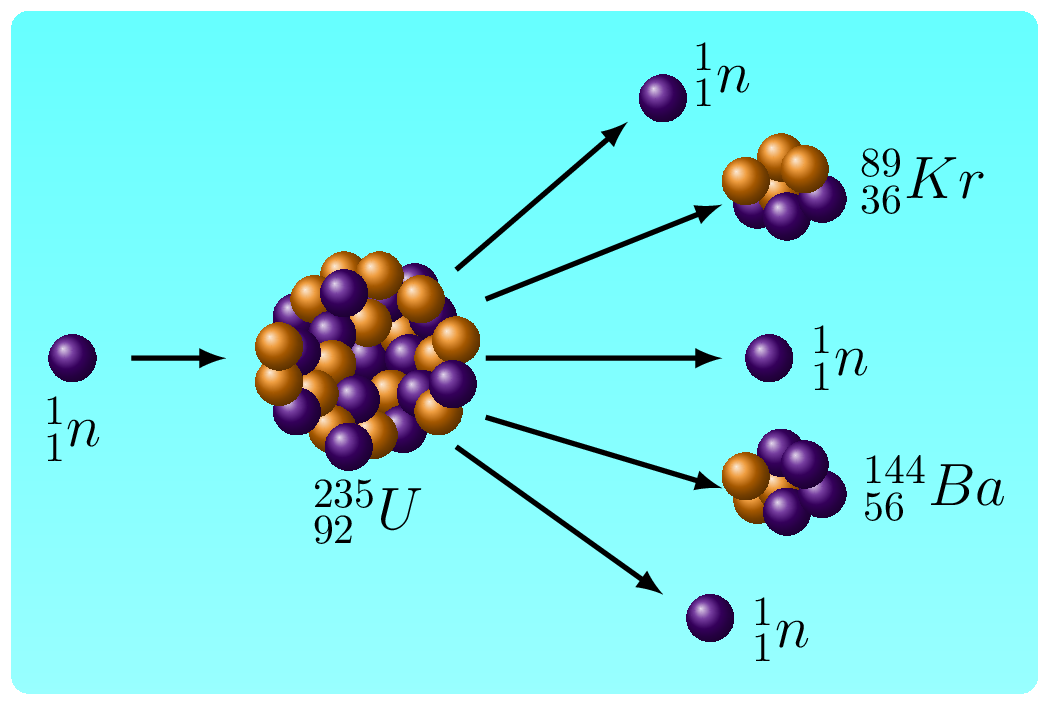
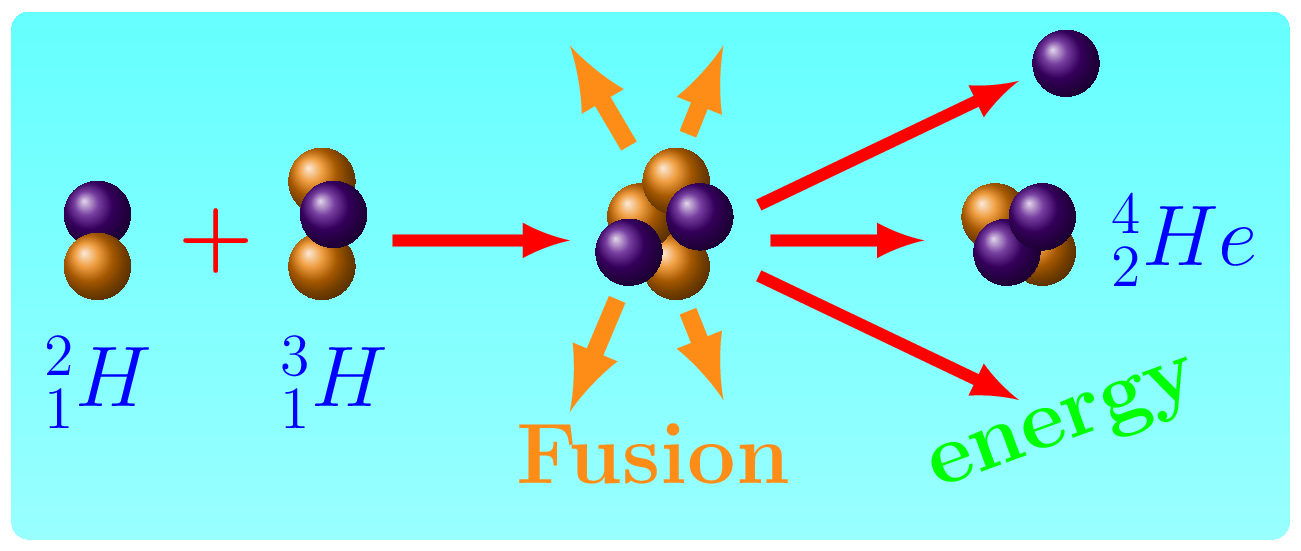
In both types of reactions, the sum of the atomic numbers of the reactants must equal the sum of the atomic numbers of the products, and the total mass of the reactants must equal the total mass of the products, according to the law of conservation of matter and energy. In fission reactions, the mass defect of the original nucleus is converted into energy, which is released as kinetic energy of the fission products and as gamma radiation. This energy can be harnessed for practical purposes, such as generating electricity in nuclear power plants, or for destructive purposes, such as in atomic bombs. Binding energy in a fusion reaction refers to the energy required to overcome the repulsive forces between positively charged nuclei and bring them close enough together to form a heavier nucleus through a fusion process. When the nuclei are close enough, they can form a single nucleus through the strong force, releasing a large amount of energy in the process.
