Section 7.1 Atomic Structure
Dalton’s atomic model was proposed in 1803. It tells that
- All matter is composed of atoms, which are indivisible and indestructible.
- Atoms of a given element are identical in size, mass, and other properties.
- Atoms of different elements have different properties and masses.
- Atoms combine in simple whole-number ratios to form compounds.
In chemical reactions, atoms are rearranged to form new compounds, but no atoms are created or destroyed. Dalton’s atomic theory laid the foundation for modern atomic theory.
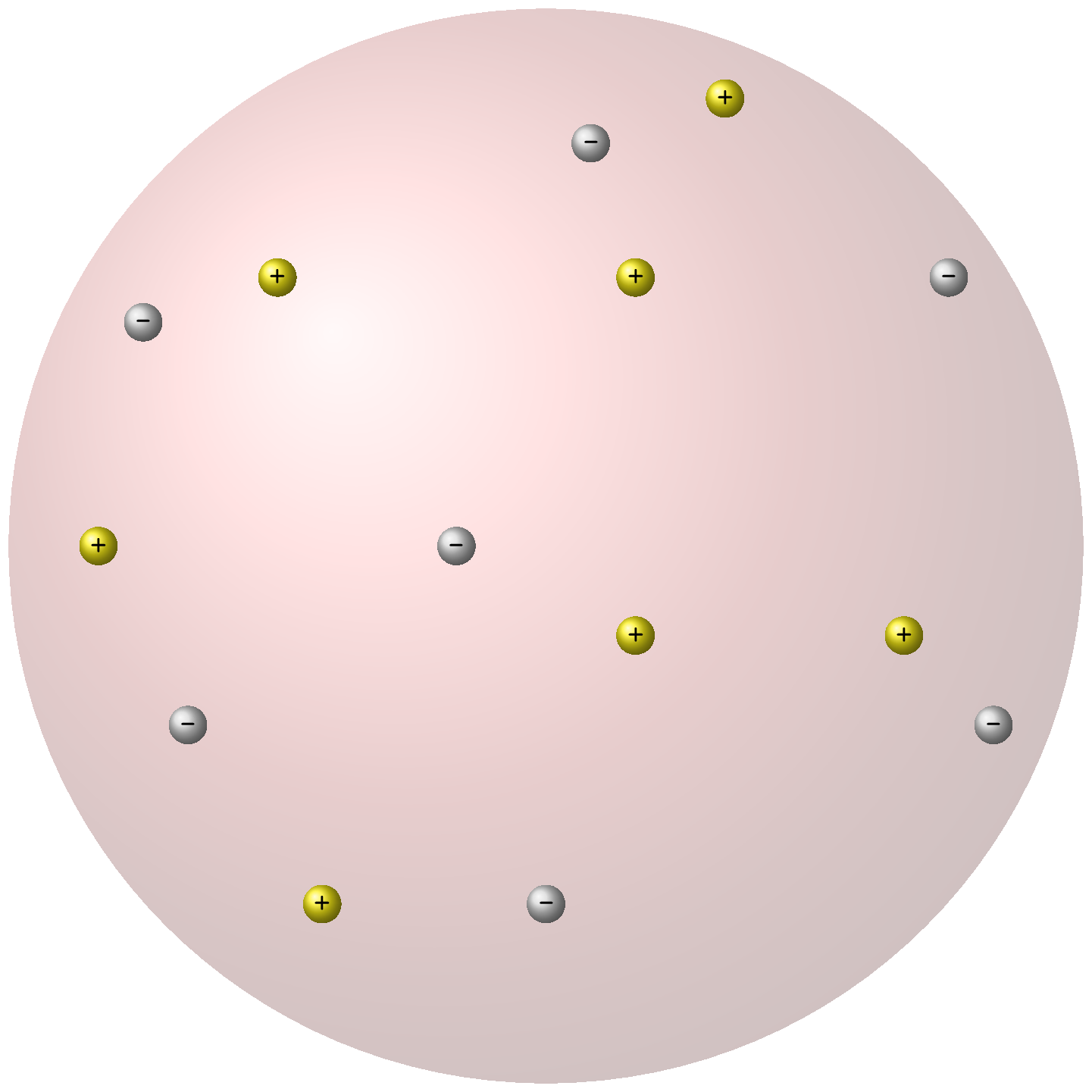
Thomson’s atomic model was proposed in 1904. It was based on his discovery of the electron, a negatively charged particle within the atom. According to Thomson’s model, the atom was a sphere of positive charge with electrons embedded within it like raisins in a pudding. Hence this model is also known as "plum pudding" model. This model was later modified by Rutherford’s atomic model.
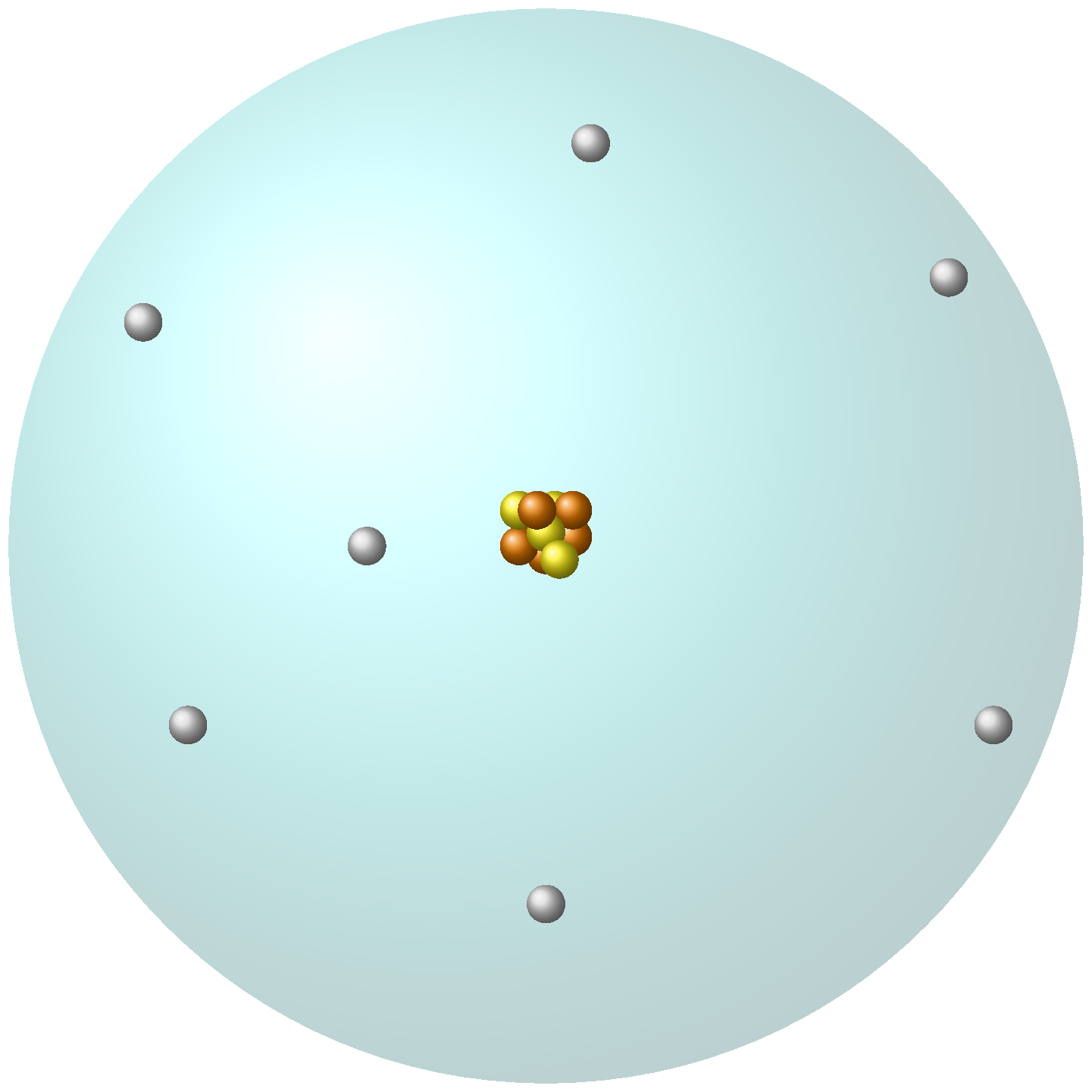
Rutherford’s atomic model, also known as the "planetary" model, was proposed in 1911 based on his gold foil experiment. The experiment involved firing alpha particles at a thin sheet of gold foil and observing their deflection patterns using a screen. The results showed that most of the alpha particles passed straight through the foil, but a small percentage were deflected at large angles, suggesting the presence of a heavy and dense nucleus at the center of the atom. Rutherford’s atomic model proposed that an atom consists of a small, dense, positively charged nucleus at its center surrounded by electrons in orbits around the nucleus. The electrons were held in their orbits by the attractive force between the positive nucleus and the negative electrons. This model explained why most alpha particles passed straight through the foil and why a small percentage were deflected at large angles. It also helped to establish the concept of the atomic nucleus and the basic structure of the atom.
Gold's Foil Experiment1
www.youtube.com/watch?v=kHaR2rsFNhg