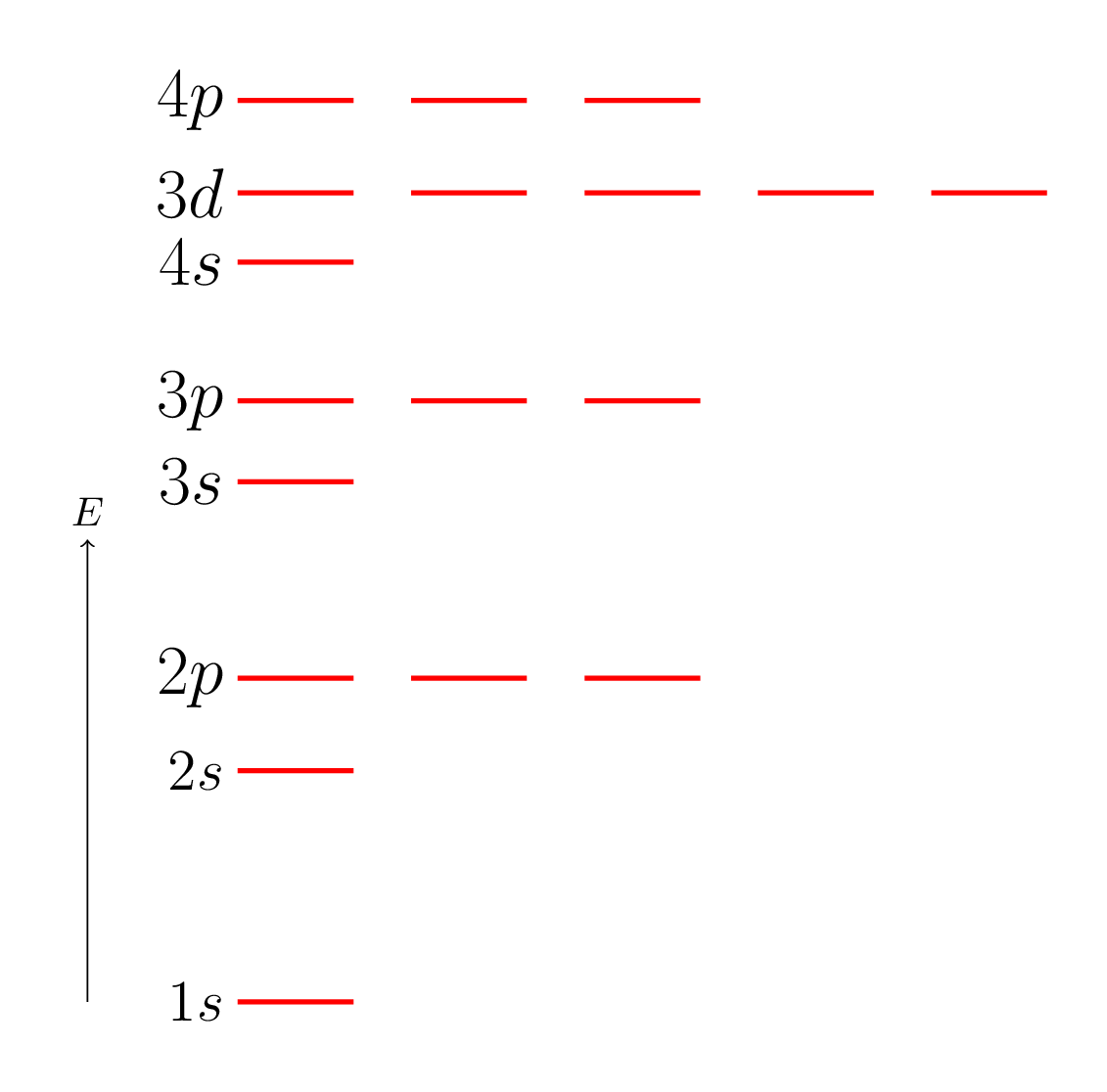Subsection 8.2.1 Electronic Configuration
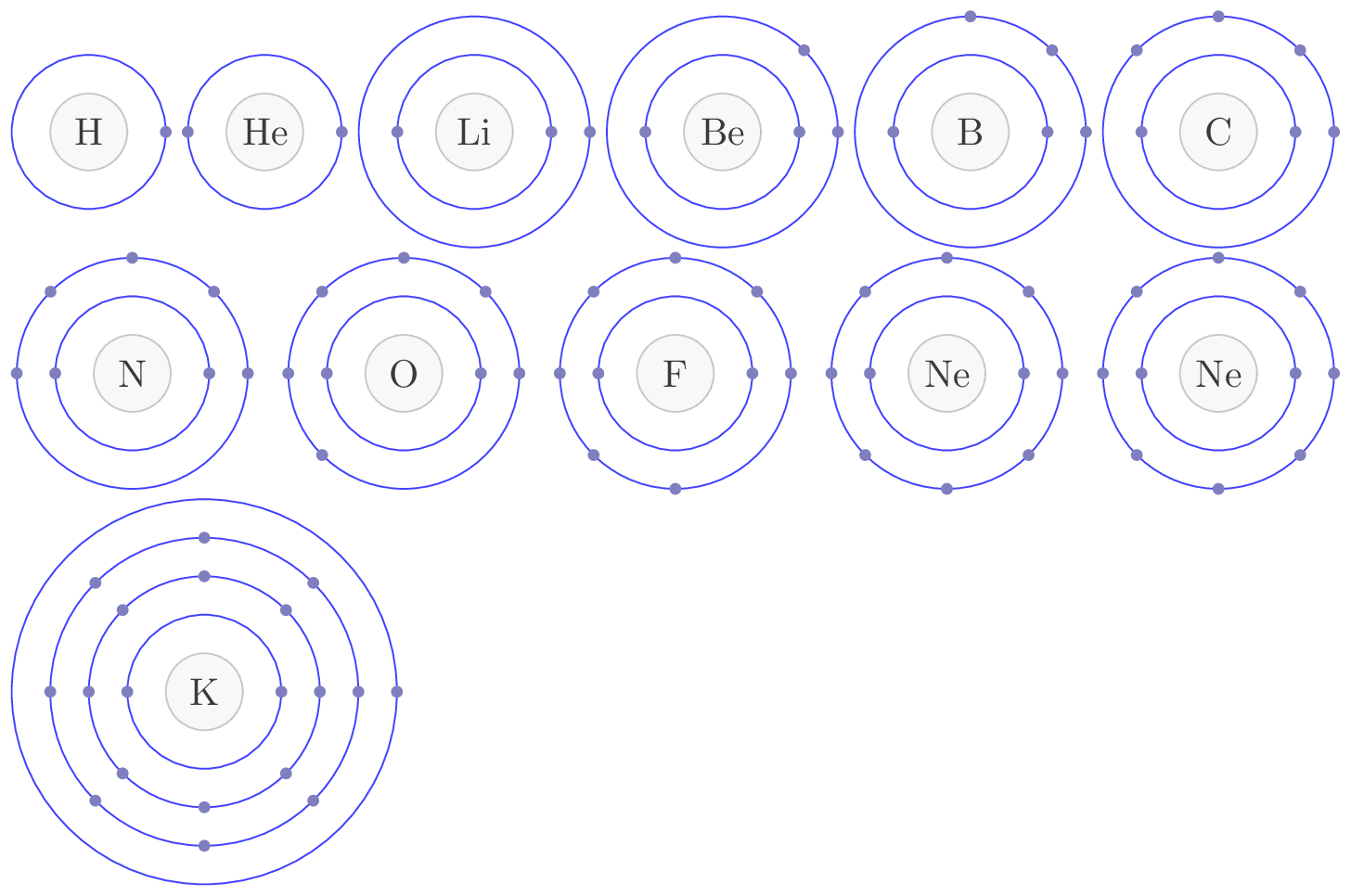
Electronic configuration is an arrangement of electrons in an atom. The electronic configuration of an atom is typically represented using the notation known as the "Aufbau principle," which states that electrons fill the lowest energy levels first, followed by successively higher energy levels. The electronic configuration of an atom is important because it determines the chemical properties and reactivity of that atom or molecule. The electronic configuration of an atom can also be represented using a diagram known as an "orbital diagram," which shows the distribution of electrons in the various sublevels of the atom. A shell is a group of electrons that occupy the same principal energy level in an atom or molecule. The shells are numbered using integers, where the first shell (or innermost shell) has a principal quantum number of 1, the second shell has a principal quantum number of 2, and so on. The \(1s, 2s, 2p, \cdots.\) in Figure 8.2.2.(a) 1, 2, 3, ... are the shells and s, p, d are the subshells in an atom. Each shell can hold a maximum number of electrons, which is given by \(2n^2\text{,}\) where \(n\) is the principal quantum number of the shell. A subshell is a division of a shell that describes the shape and energy of an electron’s orbit within that shell. The subshells are designated using the letters \(s, p, d, f, \) and so on. Each subshell is associated with a specific angular momentum quantum number, or azimuthal quantum number, which is given by an integer value ranging from \(0\) to \(n-1\text{,}\) where \(n\) is the principal quantum number of the shell. The \(s\) subshell is spherical in shape and can hold up to two electrons, the \(p\) subshell has a dumbbell or peanut shape and can hold up to six electrons, the d subshell has a more complex shape and can hold up to 10 electrons, and the f subshell has an even more complex shape and can hold up to 14 electrons. Other subshells exist for higher principal quantum numbers, but they are less commonly encountered in everyday chemistry. In summary, a shell is a group of electrons that occupy the same principal energy level, while a subshell is a division of a shell that describes the shape and energy of an electron’s orbit within that shell. The electronic configuration of an atom is typically written using the format:
\begin{equation*}
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6 5s^2 4d^{10} 5p^6 6s^2 4f^{14} 5d^{10} 6p^6 7s^2 5f^{14} 6d^{10}.
\end{equation*}