Section 10.1 Ozone
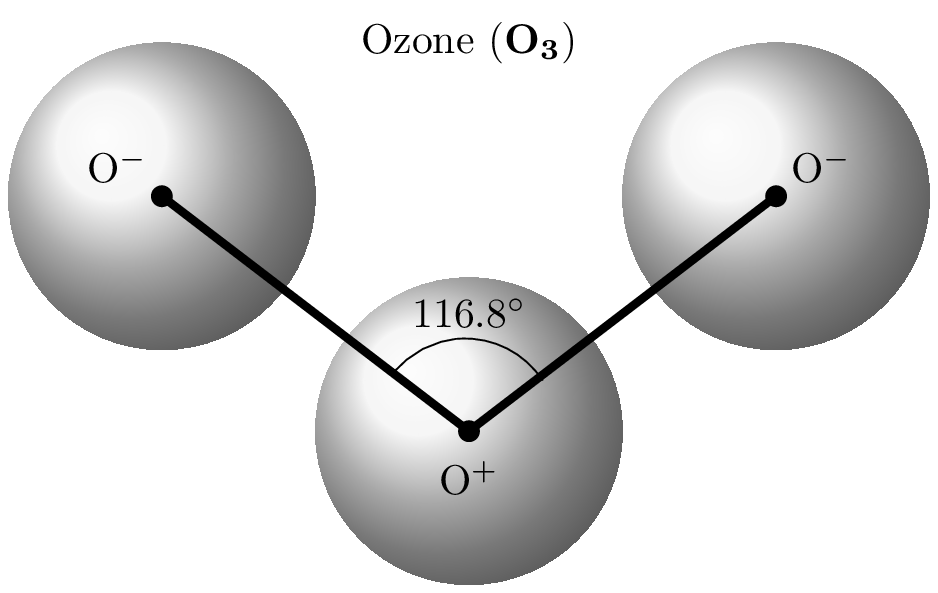
Ozone is a molecule composed of three oxygen atoms \((O_3)\) and is an important component of the Earth’s atmosphere. It is a highly reactive gas and is often referred to as a reactive oxygen species. Ozone absorbs and filters harmful ultraviolet (UV) radiation coming from the sun to enter earth troposhphere. UV radiation can cause skin cancer and other health problems. The ozonosphere, also known as the ozone layer, is a region of the Earth’s stratosphere that contains a high concentration of ozone. It is located approximately 10 to 50 kilometers above the Earth’s surface. The release of chlorofluorocarbons (CFCs) and other pollutants into the atmosphere cause the depletion of the ozone layer which is a significant environmental concern.
The photodissociation of oxygen molecules \((O_2)\) by ultraviolet (UV) radiation in presence of Nitrogen molecules \((N_2)\) help form the ozone \((O_3)\) in the Earth’s atmosphere. The reaction is shown below as:
\begin{equation*}
O_2 + UV radiation \rightarrow 2\,O
\end{equation*}
The resulting oxygen atoms (O) then react with other oxygen molecules \((O_2)\) to form ozone:
\begin{equation*}
O + O_2 \rightarrow O_3
\end{equation*}
The formation of ozone is a delicate balance between the production and destruction of ozone molecules. Ozone can also be destroyed through chemical reactions involving other compounds such as nitrogen oxides (\(NO_x\)) and chlorine compounds (Cl), which can be released from human activities such as fossil fuel combustion and the use of certain chemicals.
