Subsection 9.1.1 Polar & Nonploar Molecules
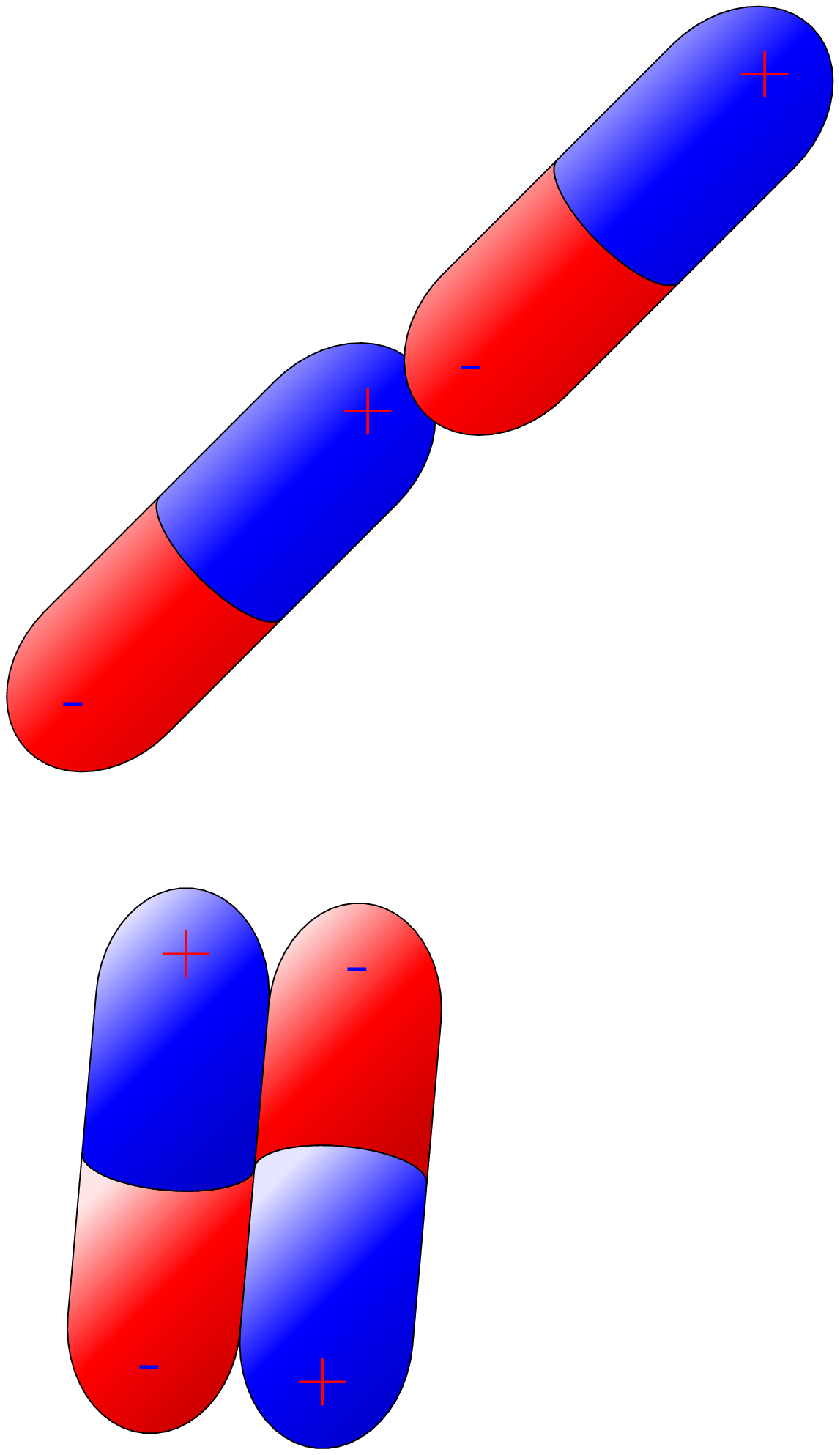
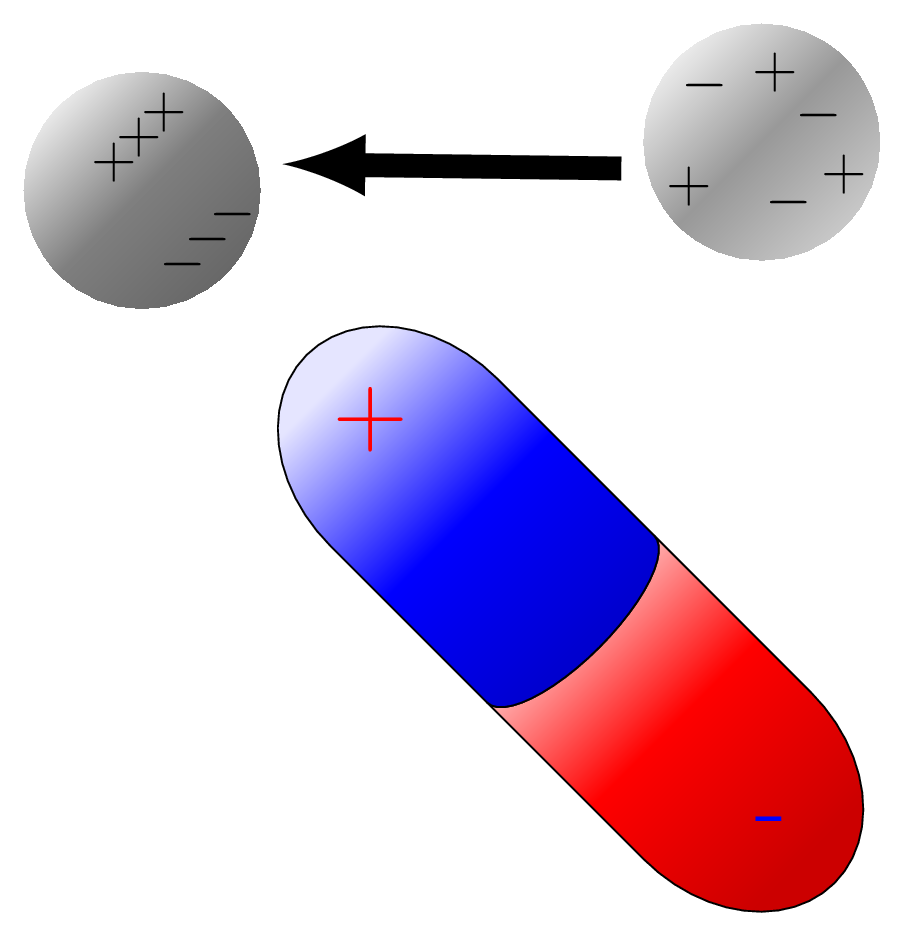
A polar molecule has none symmetrical distribution of electrons between its atoms resulting in a permanent dipole moment. This means that the molecule has a partial positive charge at one end and a partial negative charge at the other end. The polarity of a molecule is determined by the difference in electronegativity between the atoms that make up the molecule. If the electronegativity difference is large enough, the molecule will be polar. Examples of polar molecules include water \((H_2O)\text{,}\) ammonia \((NH_3)\text{,}\) and hydrogen chloride \((HCl)\text{.}\) Polar molecules are important in many biological and chemical processes, including the formation of hydrogen bonds, the solubility of molecules in water, and the function of enzymes. Polar molecules attract each other due to the presence of partial positive and partial negative charges on opposite ends of the molecule. This attraction is known as a dipole-dipole interaction [Figure 9.1.2.(a)].
A nonpolar molecule has no separation of charge, meaning that the electrons are shared equally between the atoms in the molecule. This occurs when the electronegativity difference between the atoms in the molecule is zero or very small. Examples of nonpolar molecules include: carbon dioxide \((CO_2)\text{,}\) methane \((CH_4)\text{,}\) ethane \((C_2H_6)\text{,}\) nitrogen gas \((N_2)\text{,}\) oxygen gas \((O_2)\text{,}\) benzene \((C_6H_6)\text{.}\) These molecules do not have a positive or negative pole, which means that they do not have a permanent dipole moment. Nonpolar molecules are generally less reactive than polar molecules and have lower melting and boiling points [Figure 9.1.2.(b)].
Nonpolar molecules however, interact through weak van der Waals forces, which are caused by temporary fluctuations in electron density that create a temporary dipole moment. In contrast, partial positive and negative charges on different parts of the polar molecule allow polar molecules to form strong electrostatic attractions with other polar or nonpolar molecules or ions [Figure 9.1.2.(c)].
"Like dissolves like" states that substances with similar polarities tend to dissolve in each other. In other words, a solvent will dissolve a solute if solute molecules have samilar charge distribution. Polar molecules have an uneven distribution of electrons and have a partial positive at one side of the molecule and partial negative charge on another side. Nonpolar molecules have an even distribution of electrons and do not have a net electrical charge. When a polar solute is added to a polar solvent, the polar molecules of the solute interact with the polar molecules of the solvent through intermolecular forces, such as hydrogen bonding or dipole-dipole interactions. This makes it easier for the solute to dissolve in the solvent. Similarly, when a nonpolar solute is added to a nonpolar solvent, the nonpolar molecules of the solute interact with the nonpolar molecules of the solvent through van der Waals forces, making it easier for the solute to dissolve in the solvent. On the other hand, if a polar solute is added to a nonpolar solvent, or vice versa, the polar and nonpolar molecules do not interact as easily, and the solute may not dissolve in the solvent. For example: sugar (a polar solute) does not dissolve well in oil (a nonpolar solvent) because their polarities do not match. Oil (a nonpolar solute) does not dissolve well in water (a polar solvent) because their polarities do not match. Water can be an effective cleaning agent for removing certain types of dirt, such as dust or mud, from surfaces like floors, walls, or furniture. However, if the dirt is greasy or oily, water alone may not be enough to remove it, and additional cleaning agents or solvents (such as soap) may be needed.
