Subsection 7.2.1 Radioactivity
Radioactivity is the process of spontaneous decay of an atomic nucleus. In this process, an unstable nucleus breaks apart into smaller and more stable nuclei. This process releases energy in the form of ionizing radiation, including alpha particles, beta particles, and gamma rays. Some radioactive isotopes are naturally occurring, while others can be produced artificially, for example, in nuclear reactors. Radioactivity plays many applications in medicine (e.g., medical imaging, cancer treatment), energy production (e.g., nuclear power plants), and research (e.g., dating of rocks and artifacts). Since radiactivity releases harmful radiation along with energy, it may cause harmful effect to health. Hence, it could be handled very carefully and responsibly. Radioactivity occurs due to the weak nuclear force, which is responsible for beta decay, where a neutron in the nucleus decays into a proton, an electron, and an electron antineutrino. Due to the weak nuclear force a neutron is converted into a proton by emitting beta particle which changes the atomic number of the atom and the identity of the element. However, radioactivities like alpha decay and gamma decay are not directly related to the weak nuclear force. Alpha decay is caused by the strong nuclear force, which holds the nucleus together, while gamma decay is a result of a change in the energy level of the nucleus.
In radioactive decay process different kinds of elements form due to emission of different particles or rays such as
- Alpha (\(\alpha\)) particle emission: \(\alpha\) particle is a helium nucleus and is positively charged particle. In \(\alpha\) decay a nucleus emits an alpha particle, which consists of two protons and two neutrons. For example: thorium (Th) decayes into radium (Ra) by emitting alpha particle:\begin{equation*} {}_{90}^{232}Th \longrightarrow {}_{88}^{228}Ra + {}_2^{4}He \end{equation*}This results in a decrease in the atomic number of the nucleus by two and transforms the nucleus into a different element. Alpha decay occurs when the nucleus has too many protons and is not stable, and it releases energy in the form of the alpha particle to reach a more stable configuration. Alpha particles have a large mass and a low velocity, and they can be stopped by a sheet of paper or a few centimeters of air Figure 7.2.2.
- Beta (\(\beta\)) particle emission: \(\beta\) particle is similar to electron and hence holds negative charge. In \(\beta\) decay a nucleus emits a high-energy electron or positron, also known as a beta particle. This results in a change in the number of protons in the nucleus and transforms the nucleus into a different element. For example: carbon (C-14) decayes into nitrogen (N-14) by emitting beta particle:\begin{equation*} {}_{6}^{14}C \longrightarrow {}_{7}^{14}N + {}_{-1}^{0}e \end{equation*}Similarly, neutron turns into proton in beta decay:\begin{equation*} {}_{0}^{1}n \longrightarrow {}_{1}^{1}p + {}_{-1}^{0}e \end{equation*}There are two types of beta decay: beta minus decay (emission of an electron) and beta plus decay (emission of a positron). In beta minus decay, a neutron in the nucleus decays into a proton, an electron, and an electron antineutrino. In beta plus decay, a proton decays into a neutron, a positron, and an electron neutrino. Beta decay is one of the three common types of radioactive decay, along with alpha decay and gamma decay. Beta particles have a low mass and a high velocity, and they can penetrate sheets of paper and even thin sheet of aluminum foil Figure 7.2.2.
- Gamma (\(\gamma\)) rays emission: \(\gamma\) ray is an electromagnetic radiation of very high energy and is neutral to the charge. If the nucleus is unstable due to access amount of energy then it releases \(\gamma\) rays. The composition of nucleus does not change due to gamma radiation emissioin. For example: high energetic Pb turns into low energetic Pb in gamma radiation:\begin{equation*} {}_{82}^{204}Pb^* \longrightarrow {}_{82}^{204}Pb + \gamma \end{equation*}Gamma rays have no rest mass and travel with the velocity of light and hence penetrate even thick plate of lead Figure 7.2.2.
1
star symbol indicates excited lead nucleus. - Electron (\(e^{-}\)) Capture: it is another type of radioactive decay in which an inner orbital electron is captured by the nucleus and transforms into a neutron, resulting in a decrease in the atomic number of the nucleus by one and the transformation of the nucleus into a different element. For example: flourine (F) turns into oxygen (O) due to positron emission:\begin{equation*} {}_{1}^{1}p + {}_{-1}^{0}e \longrightarrow {}_{0}^{1}n + \nu \end{equation*}Another example is\begin{equation*} {}_{92}^{231}U + {}^0_{-1}e \longrightarrow {}_{91}^{231}Pa + \nu \end{equation*}The captured electron combines with a proton to form a neutron, which remains inside the nucleus. This process releases energy in the form of gamma rays or X-rays. Electron capture is different from beta decay, which involves the emission of a beta particle (an electron or a positron).
- Positron \(e^{+}\) Emission: it is also known as beta plus decay, is a type of radioactive decay in which a nucleus emits a positron, which is the antiparticle of an electron. Positron emission occurs when a nucleus has too many protons and is not stable, and it releases energy in the form of the positron to reach a more stable configuration. For example: flourine (F) turns into oxygen (O) due to positron emission:\begin{equation*} {}_{9}^{17}F \longrightarrow {}_{8}^{17}O + {}_{+1}^0e \end{equation*}The emitted positron quickly combines with an electron in the material, annihilating both particles and producing gamma rays. Positron emission is one of the three common types of radioactive decay, along with alpha decay and beta decay. In positron emission, a proton inside the nucleus decays into a neutron, a positron, and an electron neutrino. This results in a decrease in the atomic number of the nucleus by one and the transformation of the nucleus into a different element.
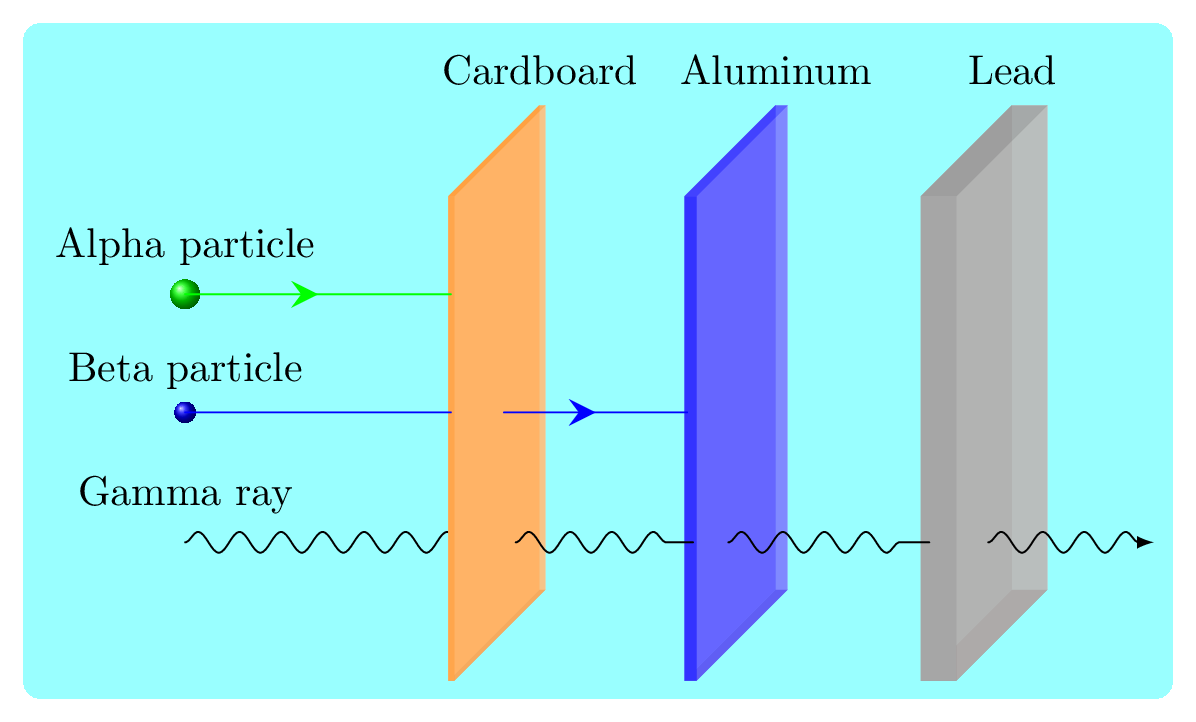
Strength of alpha particles, beta particles, and gamma raysSubsubsection 7.2.1.1 Half-Life
The rate at which a radioactive isotope disintegrates is known as its half-life, which is the time it takes for half of the radioactive material to decay. The half-life of a radioactive isotope determines its stability and the length of time it can be used for various purposes, such as medical treatments or energy production. The Figure 7.2.3 representing the amount or number of atoms in a radioactive substance remains every after one half-life. For example, in second figure of Figure 7.2.3, half-life of any one radiactive substance is 50 years, so half of the total number of atoms or its half amount disintegrate (decay) into new substances in every one half life.
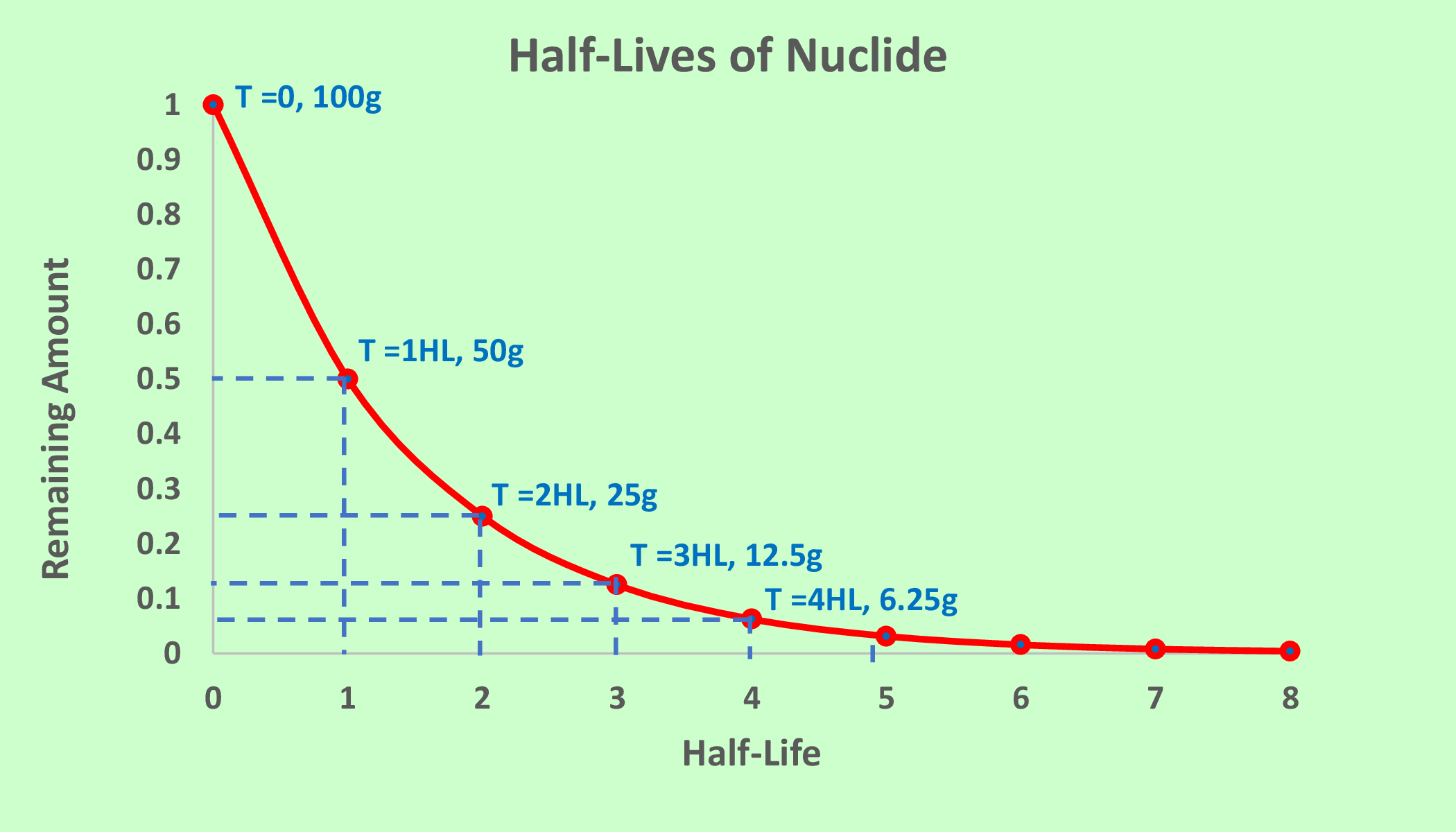
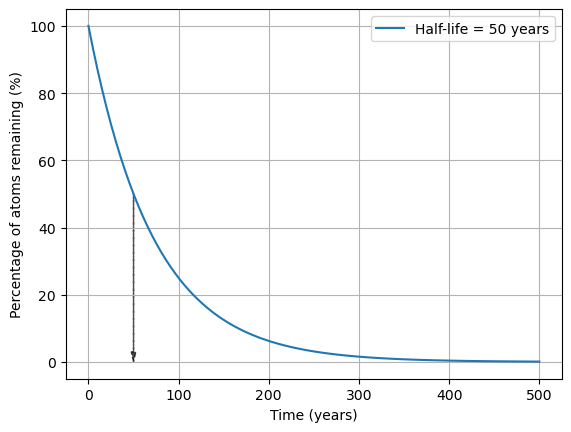
The remaining amount of substance \(N\) can be determined by the following formula
\begin{equation}
N = \left(\frac{1}{2}\right)^{n}\times (N_o)\tag{7.2.2}
\end{equation}
Here, \(N_o\) is initial concentration of the substance, and \(n\) is the number of half-lives.
Subsubsection 7.2.1.2 Carbon Dating
Carbon dating is a method of determining the age of an object containing organic material by measuring the amount of carbon-14 isotopes contained in the object. Carbon-14 is a radioactive isotope of carbon that is formed in the Earth’s upper atmosphere through the interaction of cosmic rays with nitrogen atoms. The amount of carbon-14 in the atmosphere has remained constant for thousands of years, so the ratio of carbon-14 to carbon-12 in a sample can be used to determine how long it has been since the death of the organism from which the sample was taken. The carbon-14 in a sample decays over time, and the amount remaining can be measured with a specialized machine called a mass spectrometer. The age of the sample can then be calculated based on the known decay rate of carbon-14. This method is typically used to date organic materials that are less than 50,000 years old, such as archaeological artifacts, fossils, and recent organic samples. In the upper atmosphere Cosmic rays which was primarly protons turns into neutrons. These neutrons react with nitrogen to produce carbon-14.
\begin{equation*}
{}_{7}^{14}N + {}_{0}^{1}n \longrightarrow {}_{6}^{14}C + {}_{1}^{1}H
\end{equation*}
The carbon-14 then reacts with oxygen to to become \(CO_2\) gas. Plants consume this \(CO_2\) in photosynthesis process. Later animals eat plant and stores these C-14 atoms in their body. In beta decay process C-14 keeps turning into nitrogen such as
\begin{equation*}
{}_{6}^{14}C \longrightarrow {}_{7}^{14}N + {}_{-1}^0e
\end{equation*}
The ratio of carbon-14 to carbon-12 is roughly constant because the organism continuously replaces carbon-14 as it decays. However, after the organism dies, the carbon-14 in its tissues begins to decay, and the carbon-14 to carbon-12 ratio begins to change. By measuring the carbon-14 to carbon-12 ratio in a sample and comparing it to the known decay rate of carbon-14, scientists can determine how long it has been since the sample died and estimate its age.
