Chapter 9 Crystals and Solutions
According to the modern theory of atoms, solids are materials that consist of closely packed atoms or molecules that are held together by strong intermolecular forces. These intermolecular forces are created by the interactions between the electrons in the outermost energy levels or orbitals of adjacent atoms. In solids, the atoms,ions, or molecules are arranged in a specific pattern, known as a crystal lattice, which gives the solid its characteristic shape and structure. The arrangement of atoms in the crystal lattice determines many of the properties of the solid, such as its density, strength, and melting point. The electrons in the outermost energy levels of the atoms in a solid are not free to move around as they are in metals, but instead are closely bound to their respective atoms. However, in some solids, such as semiconductors and metals, some electrons are free to move within the crystal lattice, and these materials can conduct electricity and heat.
The behavior of solids under different conditions, such as temperature and pressure, can be explained by the modern theory of atoms. For example, the thermal expansion of solids occurs because as the temperature of the solid increases, the atoms or molecules in the crystal lattice vibrate more rapidly, causing the material to expand. The strength of solids can also be explained by this theory, as the intermolecular forces between atoms determine the material’s ability to withstand external forces.
There are basic two types of solids:
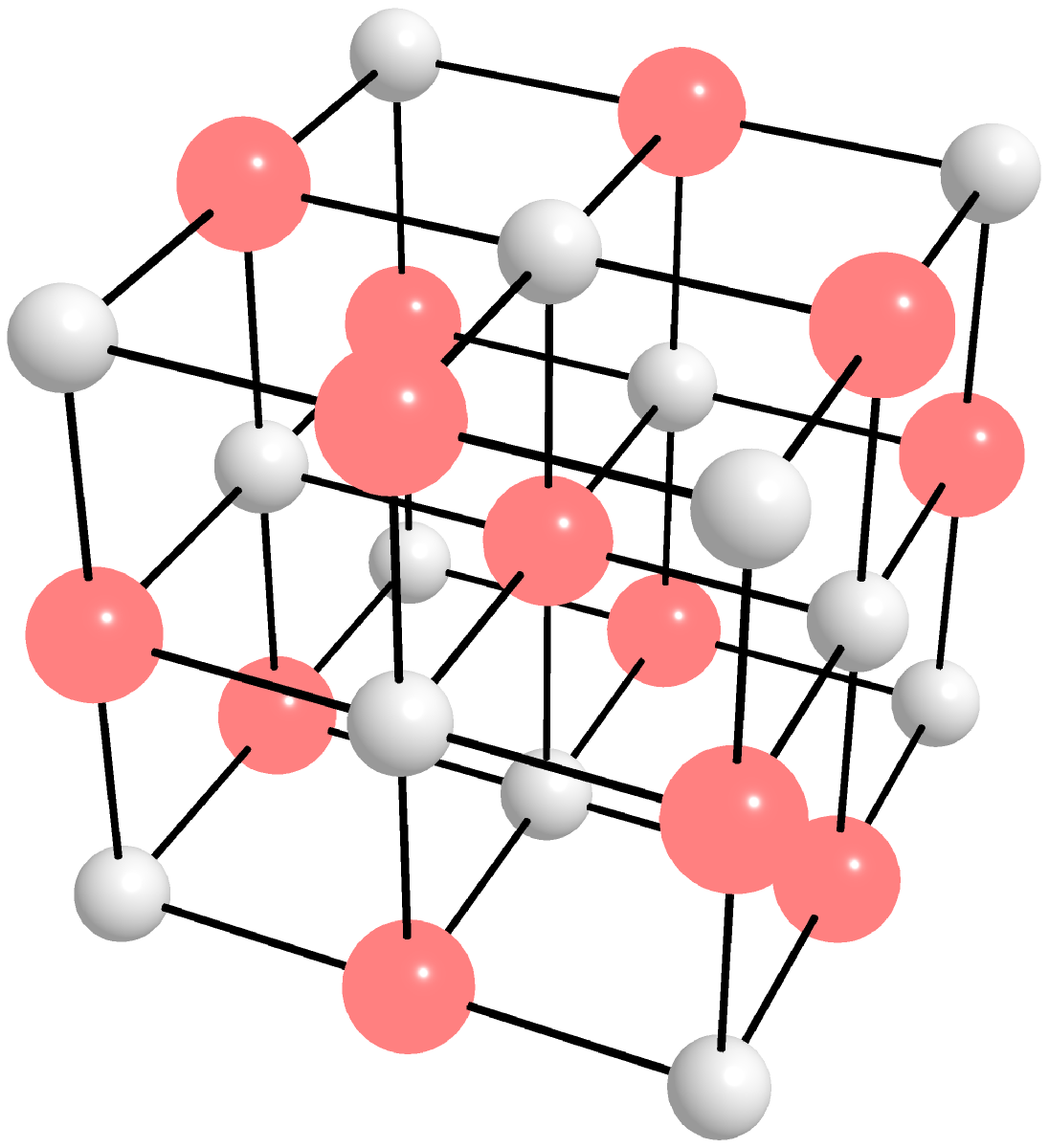
- Crystalline Solids: These are solids with a regular, repeating pattern of atoms, molecules or ions arranged in a three-dimensional lattice structure. The lattice structure gives the solid its characteristic properties, such as melting point, hardness, and conductivity. Crystalline solids can be further classified into two subtypes: molecular crystals, where the repeating units are molecules, and atomic crystals, where the repeating units are atoms. Examples of atomic crystals are diamond, salt, iron, copper, etc. Examples of molecular crystals are ice, quartz, sugar, and organic crystals such as caffeine, aspirin, and cholesterol, etc. Figure 9.0.1.(a)
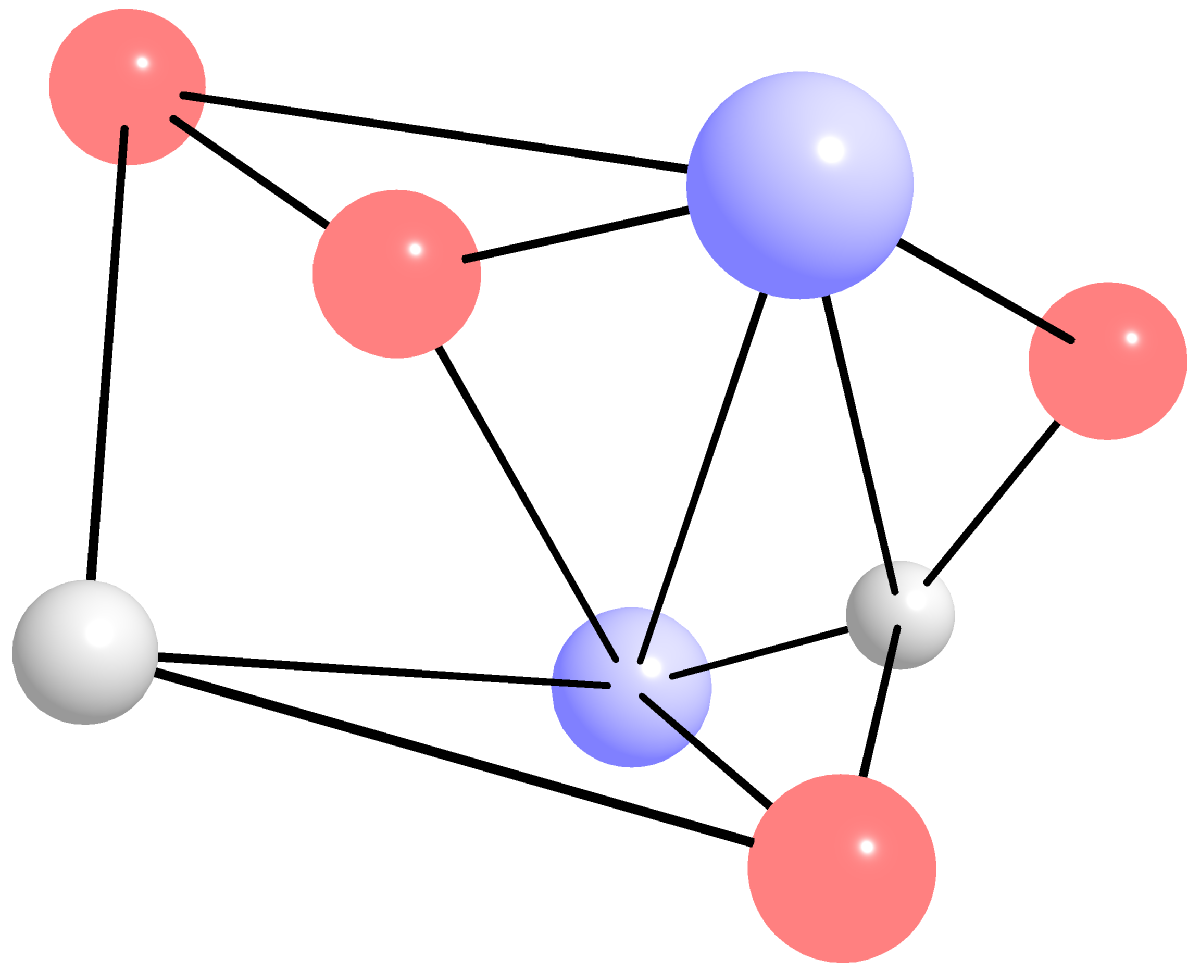
- Amorphous Solids: These are solids without a regular lattice structure. In Greek amorphous means "without form". The arrangement of particles in amorphous solids is random and disordered. Examples include glass, rubber, and plastic. Glass is amorphous because its atoms or molecules do not have a regular or periodic arrangement. Unlike crystalline solids, which have a well-defined lattice structure, the arrangement of particles in glass is random and disordered. The amorphous nature of glass arises due to the way it is formed. Glass is typically made by cooling a molten liquid too quickly for its atoms or molecules to arrange themselves into a regular lattice structure. As a result, the particles in glass are frozen in a random arrangement, leading to an amorphous solid. This amorphous nature of glass gives it some unique properties, such as being transparent, brittle, and having a relatively low melting point compared to crystalline solids. The lack of a defined structure also means that glass can be easily molded into a variety of shapes, making it useful in many applications such as windows, lenses, and optical fibers. However, the lack of a defined structure also makes glass more prone to fractures and cracking, as the random arrangement of particles makes it difficult for the material to dissipate stress and strain. Figure 9.0.1.(b)
However, there are other two types of solids also exist such as Polycrystalline Solids: These are materials made up of many small crystalline regions or grains that are oriented in different directions. Polycrystalline solids often have improved mechanical properties over single crystals, as the different grain boundaries can strengthen the material. Composite Solids: These are materials made up of a combination of two or more different types of solids. The different components can have distinct properties, resulting in a material with a unique set of properties. Examples of composite solids include concrete, which is made up of cement and aggregate, and reinforced plastics, which are made up of a plastic matrix with embedded fibers for added strength.
These four types of solids can also be classified based on their electrical and magnetic properties, as well as their response to temperature changes, deformation, and other external stimuli. Understanding the different types of solids is essential for the design and development of new materials with specific properties and applications.
