Subsection 7.1.4 Bohr’s Model of Hydrogen Atom
The Bohr model of the atom is a simplified representation of the structure of an atom proposed by Niels Bohr in 1913. According to the Bohr model, an atom consists of a central nucleus composed of positively charged protons and neutral neutrons surrounded by electrons in shells or orbits. The electrons occupy discrete energy levels and move in circular orbits around the nucleus, and the energy of an electron depends on its distance from the nucleus. The quantized orbits correspond to specific energy levels, and the transition of an electron from one energy level to another results in the emission or absorption of electromagnetic radiation, which is observed as a spectral line. The Bohr model helps explain the emission and absorption spectra of atoms and laid the foundation for the development of quantum mechanics. The Bohr model is used to describe the structure of hydrogen energy levels. The Figure 7.1.5.(a) represents shell structure, where each shell is associated with principal quantum number \(n\text{.}\) The amount of energy in each level is reported in eV, and the maxiumum energy is the ionization energy of 13.6 eV. The movement of electrons between these energy levels produces a spectrum.
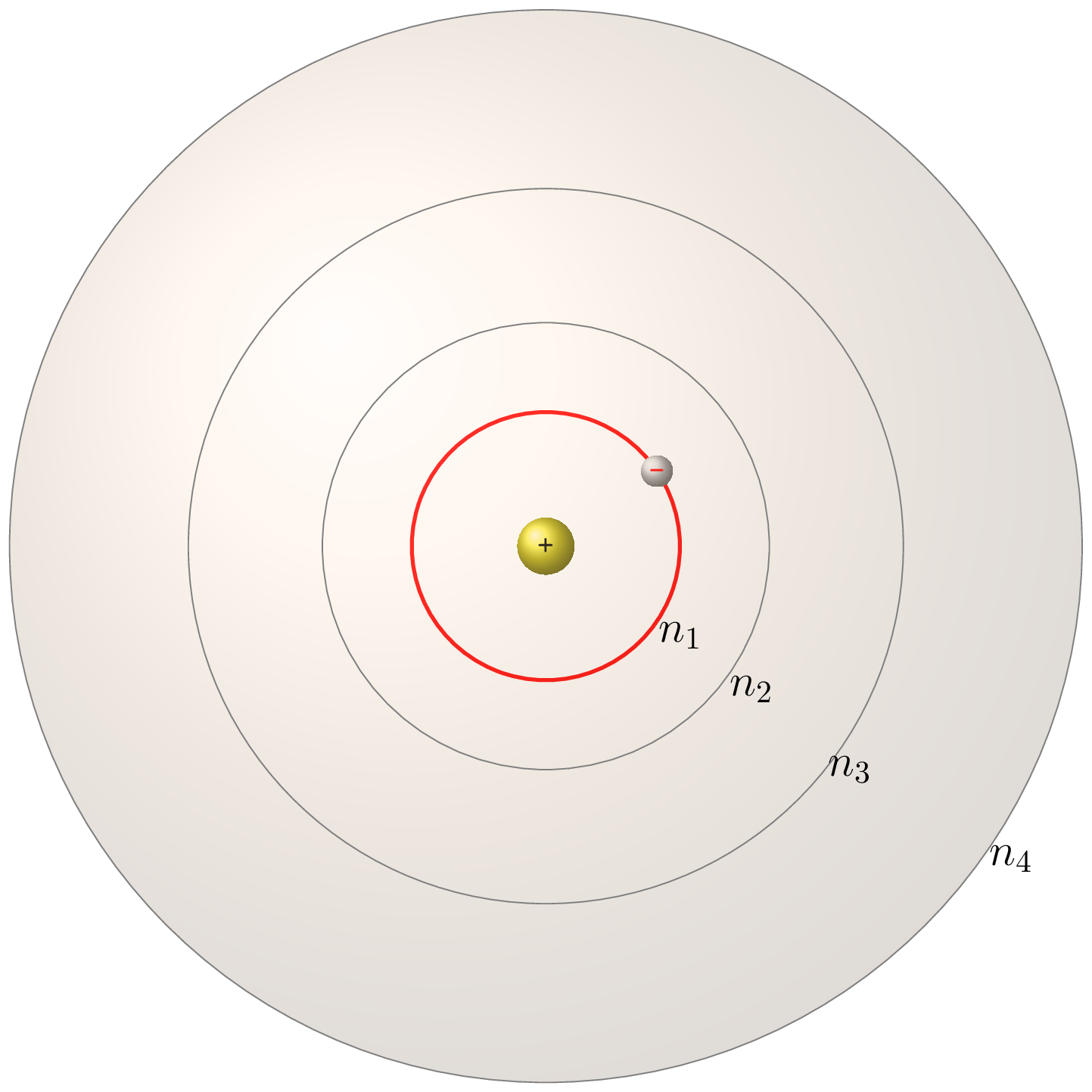
The allowed value of the atomic radius is given by
\begin{equation}
r(n) =n^2\times r(1)\tag{7.1.3}
\end{equation}
Where, \(n\) is a positive integer, \(r(1)\) is the smallest allowed radius for the hydrogen atom also known as the Bohr’s radius, \(r(1) = 0.529\times 10^{-10} \,m\text{.}\) Bohr calculated the energy of an electron in the \(n^{th}\) level of hydrogen atom by using the formula given below
\begin{equation}
E(n) =-\frac{1}{n^2} \times 13.6 \,eV\tag{7.1.4}
\end{equation}
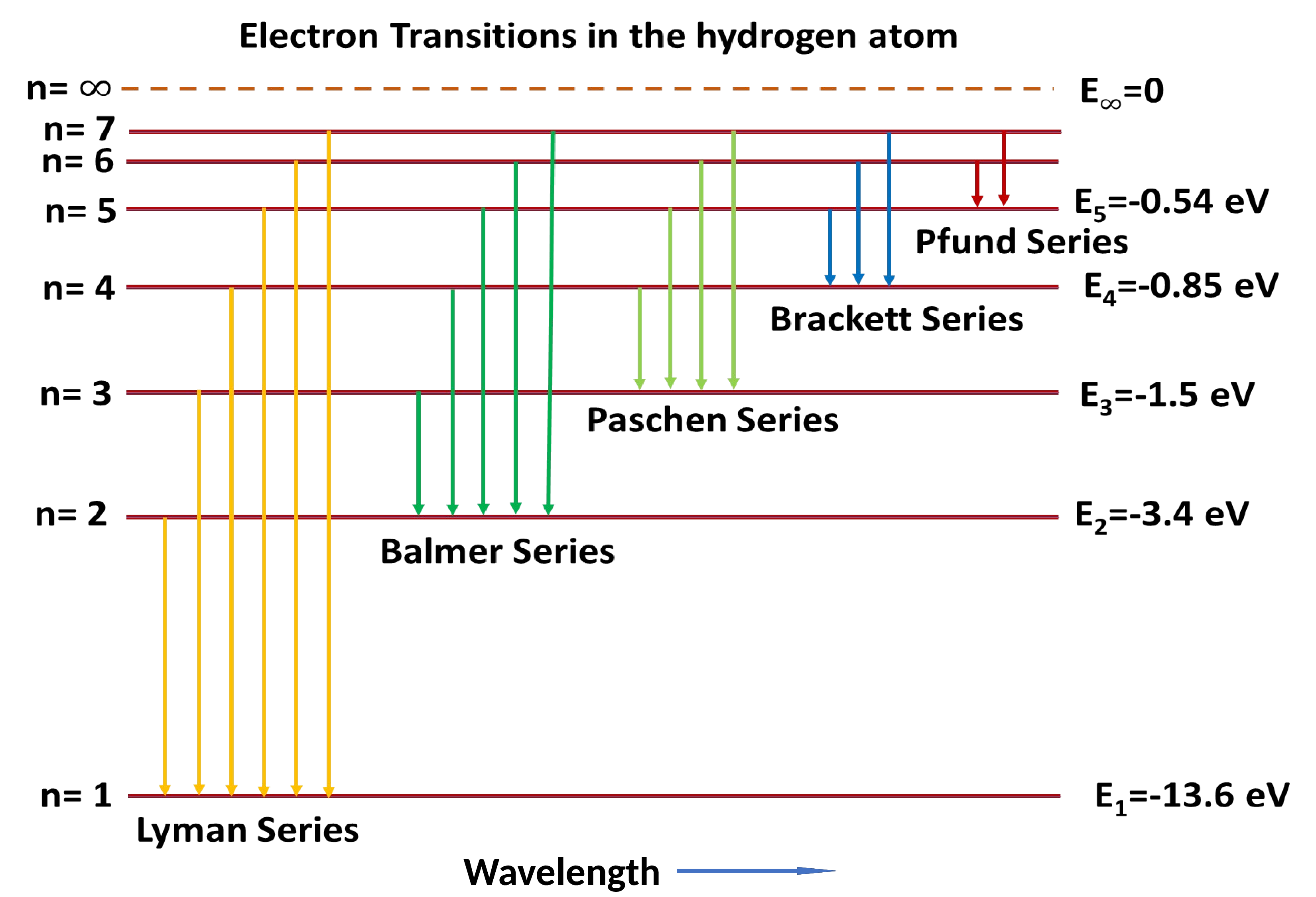
In Figure 7.1.5.(b) the numbers on the left side represent energy levels with the lowest energy level at the bottom and on right side representing their corresponding values. The arrows represent all possible transitions of electron from one energy level to another. Each group of transitions is given the name of the scientist who identified their origin. Longer arrow means more energy is released during transition and hence the light of shorter wavelength is being emitted. The Balmer series releases light in the visible region of the electromagnetic spectrum. The Lyman series, with longer arrows, requires the higher energy of the UV region. The Paschen, Brackett series, and Pfund series with shorter arrows require the lower energy of the IR region. According to Bohr model, Lyman series appears when electrons jump from higher energy levels \((n_h = 2,3,4,5,6,\cdots)\) to \(n_l = 1\) energy state. Balmer series appears when electrons jump from higher energy levels \((n_h = 3,4,5,6,\cdots)\) to \(n_l = 2\) energy state, and so on.
